1. Introduction
According to the World Health Organization (WHO), 6.3 million new cases of tuberculosis were recorded worldwide in 2016. Most cases occur in low socio-economic countries; the African region was affected by 25% of cases [1]. In the same year, new cases of tuberculosis in children under 15 years old represented around 6.9% of all cases in the world [1]. In Burkina Faso, pulmonary tuberculosis is the most common form; It accounted for 83% of all reported cases in 2016 [2]. In 2015, the number of tuberculosis-related deaths among people under 15 years was estimated at 239,000 and cases of co-infection with HIV accounted for 17% [3]. As a result of its relative frailty and its inescapable contact with adults, the child is a favorite place for tuberculosis. However, the diagnosis of childhood tuberculosis, even in its pulmonary form, remains difficult, especially in developing countries due to lack of efficient means. The diagnosis is usually based on a non-specific arguments beam, such as concept of contagion, signs of tuberculous impregnation, chest X-ray images, and tuberculin intradermoreaction.
The aim of this study was to describe the epidemiological, clinical and paraclinical aspects of childhood tuberculosis in the pediatric department of Souro Sanou University Hospital Center in Bobo- Dioulasso.
2. Patients and Methods
It was a cross-sectional descriptive study with prospective collection. It ran from January 1st to December 31st 2016, a 12-month recruitment period.
Were included in this study, children aged from 0 to 15 years old, in whom the diagnosis of tuberculosis was made during the period of study in the Department of Pediatrics. This diagnostic was based on anamnestic arguments (concept of contagion, signs of tuberculous impregnation, chronicity of signs, known HIV infection), clinical (pleuropulmonary signs, fistulized lymphadenopathy, spinal hump...) and laboratory (AFB in sputum, inflammatory syndrome, evocative chest X-ray, ultrasound...).
Patients were included consecutively according to the rate of admission during the collection period. The consent of parents or legal guardians was required before any inclusion.
The clinical and para-clinical data of each patient were collected daily at the level of the different hospitalization services of the pediatric department of Souro Sanou Hospital. The data were collected by apretested and validated questionnaire. They have been entered on the EPI data 3.1 software. The analysis was performed using STATA 12. The existence of statistical links between the variables of interest was investigated by Chi2test. The link was considered significant for a "p" of less than 5% (risk α).
3. Results
During the study period, 28 new cases of tuberculosis were recorded in the Department of Pediatrics of Souro Sanou Hospital, a hospital frequency of 0.2%. There was a predominance of boys (17/28), with a sex ratioof 1.5. The average age of the children was 8.3 years old with extremes of one month and 15 years. Two children were less than one year old (one month and six months; maternal story). The under five years group was eight. Ten children were between five and 10 years old and 10 others were over 10 years old; 71.4% of children over five years old. The area of residence was predominantly rural and involved 17 children. The 11 others were living in Bobo-Dioulasso.
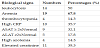
Passive smoking was found in nine children. Two children did not receive the BCG vaccine. A concept of contagion was found in 12 children; the adult contaminant was one of parents in seven of them. Three underlying chronic diseases were found. These included malnutrition in 23 children, HIV infection in five children, and sickle cell disease in two children. These morbidities were sometimes associated.
The most common clinical signs were long-term fever, decreased play activities, chronic cough and night sweats. The other signs were often suggestive of the clinical form of tuberculosis (lymphadenopathies, spinal gibbosity, meningeal syndrome, etc.). Signs were often associated in the same patient. Table 1 is a summary of the clinical signs observed in children.
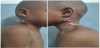
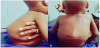
Figures 1, 2 and 3 are photographs of children of the series, respectively showing chest X-ray, fistulized lymphadenopathy, and spinal gibbosity.
- a vast cavern of the lingula (star) with a drainage bronchus (double arrow)
- a right apical infiltrate (arrow)
- and a left basal milial aspect (arrowhead).
The most common biological abnormalities were anemia and leukocytosis.Table 2 gives a summary of the biological abnormalities found in the patients.
Direct sputum examination was positive in only two children. These were tuberculosis cases with isolated pulmonary localization.
The GeneXpert test/ MTB / RIF performed in nine children was negative.
Chest X-ray was abnormal in 21 children. Pulmonary lesions were varied and dominated by parenchymal infiltrates. Cellular condensation was found in 32.1% of patients. Four cases of military radiological were observed. Table 3 illustrates the different lesions and their frequencies.
Abdominal ultrasonography was performed in 11 patients, showing numerous abnormalities, the most common being ascites and deep lymphadenopathy. These anomalies were often associated. Table 4 gives the synthesis of different abnormalities found in abdominal ultrasound.
In total, tuberculosis was unifocal in 13 children (46.4%) and multifocal in 15 (53.6%). The most frequent locations were lung (nine cases including seven associated with pleural involvement), lymph nodes and abdomen. Figure 4 illustrates different locations found.
4. Discussion
4.1 Epidemiologyand context
Twenty-eight new cases were reported during our study. A hospital study in Togo reported an annual average of 18 cases [4]. The hospital frequency of 0.2% that we found reflects a relative rarity of tuberculosis in children. Under-reporting of cases is not excluded. Indeed, the multiplicity of clinical presentations in children can lead to diagnostic errors and the use of traditional medicine, favored by mystical beliefs. This is especially true since the majority of cases came from rural areas.
Children over five were the most numerous in our series, as reported in other African studies [4-6]. Nevertheless two young infants had been infected by their mothers. Parental contagion effect was found in 58.3% of the total cases where a concept of contagion was reported. Tuberculous familial contours of children have often been reported [7,8]. Almost all children had been vaccinated with BCG, thus demonstrating already the limits of this vaccine in the prevention of tuberculosis [9,10]. Factors favoring infection such as passive smoking reported by other authors [11], as well as malnutrition have been found in some of our patents. HIV infection, for its part, is recognized as a major circumstance for the occurrence of tuberculous infection in children. The advent of HIV has led to an upsurge in tuberculosis and the combination of HIV/TB is a source of high morbidity and mortality in the pediatric population [5,12].
4.2 Clinical and para-clinical signs
The most common clinical signs were long-term fever, decreased play activities, chronic cough, and night sweats. This and other signs such as night sweats, asthenia, weight loss and so-called "tuberculous impregnation" were not always found in our patients. On the other hand, the fever concerned almost all of them (92.9%). It is reported variably by different authors [6,13]. Anemia was the most common biological abnormality even though it was not often evident in the clinical setting. Anemia is indeed common during childhood tuberculosis; it is of inflammatory type directly related to the disease, but it can be aggravated by a deficiency anemia, especially in cases of malnutrition as in up to 82.1% (23/28) of our patients.
5. Diagnostic
In the majority of cases, the diagnosis of tuberculosis was made on the basis of an amnestic, clinical and para-clinical presumptive arguments. It is the same in other works [5,6,14]. Diagnostic confirmation of childhood TB remains difficult, even in better working conditions and despite the use of powerful technologies such as PCR and the detection of interferon-γ.
6. Location
Pulmonary tuberculosis was the most common clinical form, the so-called misconception that children would mostly be extrapulmonary. This finding is shared by several authors in Africa [6,15]. However, a scarcity of bacillus forms is certainly related to the fact that tuberculosis of the child is often paucibacillary [16]. Reaching peripheral lymph nodes was the first extra pulmonary location. In fact, ganglionic tuberculosis is common in children, with a significant contribution from pathological anatomy to diagnosis [17,18].
7. Conclusion
Tuberculosis remains a public health problem in developing countries and children are not spared. Diagnostic difficulties persist despite technological progress and efforts of states and organizations in the fight against this disease. BCG remains the order of the day, but prevention of childhood tuberculosis is primarily based on the detection and early treatment of cases in adults.
Competing Interests
The authors declare that they have no competing interests.