1. Introduction
Biloma is a rare disease defined as an encapsulated collection of bile due to disruption of the biliary tree [1]. The majority of bile leaks occur secondary to traumatic or iatrogenic injury, including abdominal surgery, laparoscopic surgery, and percutaneous catheter drainage. Spontaneous perforation of the biliary tree without anprevious history of trauma or surgery is an extremely rare cause of biloma [2].
The pathophysiology of spontaneous biloma is not fully clear, but one suggested contributing factor is increased intraductal pressure due to obstructive lesions or infarctions in any part of the biliary tree. Most cases of previously reported spontaneous bilomas occurred secondary to choledocholithiasis or cholangiocarcinoma. Other rare causes described in the literature are acute cholecystitis, pancreatic adenocarcinoma, sickle cell disease and tuberculosis [3].
The complications of sclerosing cholangitis are recurrent cholangitis, cirrhosis, cholangiocarcinoma, hepatocellular carcinoma, gallbladder carcinoma, cholelithiasis, fat-soluble vitamin deficiencies and osteoporosis. Additionally, primary sclerosing cholangitis has be enassociatedwithinflammatoryboweldisease [4]. Spontaneous biloma formation with sclerosing cholangitis has not been reported before, according to our best knowledge. Here, we reported a patient with huge spontaneous biloma-related sclerosing cholangitis.
2. Case Presentation
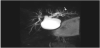
A 16-year-old female patient was admitted to our hospital with nausea, generalized abdominal pain and distention without fever or jaundice. It was learnt that she got a diagnosisof celiac disease twelve years ago, and sclerosing cholangitis four years ago.The lower gastrointestinal endoscopy and histopathology carried out to exclude inflammatory bowel disease were normal. She was taking prednisolone (5 mg/day) and azathioprine (50 mg/day) for four years. There was no history of trauma and/or abdominal surgery. Four months ago, magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) revealed distal narrowing and proximal dilatation of the common hepatic duct (CHD) and dilated intrahepatic bile ducts (IBDs). In addition, the dilatation of the left intrahepatic bile ducts was more pronounced than the right intrahepatic bile ducts (Figure 1). On physical examination, her weight was 32 kg , height was 145 cm and body massindexwas 15,2 (all of them below the 3rd percentile for her age). Her pubertaldevelopmentwas at Tanner stage 5. Thepatient's body temperaturewas 36,8 °C , herbloodpressurewas 110/60 mm/ Hg and her heart rate was 88 beatsperminute. She had distinct abdominal distention. Abdominal percussion revealed dull sounds throughout the right upper and lower quadrants. Complete blood count revealed microcytic anaemia (haemoglobin 10.6 g/dL, mean corpuscular volume 75.2 fL). Erythrocyte sedimentation rate (ESR) = 63 mm/h, and C-reactive protein (CRP) = 12.9 mg/L (range 0–5 mg/L), AST= 43 IU/L, ALT=32 IU/L, total bilirubin=0.74 mg/dl, direct bilirubin= 0.34 mg/dl, alkaline phosphatase=116 IU/L, gammaglutamyltranspeptidase (GGT) =38 IU/L, CA19-9 =32 IU/mL (0–36 IU/mL), and carcinoembryonic antigen (CEA) = 2.6ng/ml. Her renal function tests and electrolytes levels were normal.
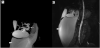
Abdominal ultrasound and MRI demonstrated a loculated fluid collection extending from the subcapsular region of the right hepatic lobe to the pelvic inlet, measuring approximately 25×12×14 cm (Figure 2). However, no stones were observed in the gallbladder or biliary tree, nor in the mass. MRCP showed prominent dilatation of the proximal common hepatic bile duct and the left intrahepatic bile duct, and a slightly dilated right intrahepatic bile duct. Additionally, the distal part of the common bile duct (CBD) could not be visualized (Figure 3A-3B).
The loculated fluid was drained percutaneously by needle aspiration. The aspirated fluid total bilirubin level = 20.8 mg/ dL and a direct bilirubin level = 6.7 mg/dL. Hence, the fluid was confirmed to be a biloma. No microorganism was detected on Gram staining. Culture and cytology of the aspirated fluid were negative. However, the same amount of liquid, by a percutaneous catheter (10-French), was reaccumulated within four days. Antibiotic treatment was initiated in order to prevent infection. Feeding was stopped and total parenteral nutrition was started to reduce bile production. Even then, approximately 10-20 ml of liquid continued to flow from the drainage daily. Cholestasis developed on the tenth day of hospitalization (ALP=153 IU/L, GGT=398 IU/L, total bilirubin 5.72 mg/dl, and direct bilirubin 4.07 mg/dl). It was considered that the huge biloma could be compressing the CBD and than endoscopic retrograde cholangio-pancreatography (ERCP) was performed. ERCP showed compression of the CBD by a huge biloma. During the ERCP, we carried out an endoscopic sphincterotomy and a 10 Fr stent was placed into the CBD, to provide the decompression of CBD and reduce the intraductal pressure (Figure 4A-4B). Serum ALP, GGT and bilirubin levels subsequently decreased to normal levels, and the bile drainage ceased after two days. The percutaneous drainage catheter was removed, and the patient was discharged with full recovery. No recurrence-related clinic encountered during her 3 months of followup.
3. Discussion
The first case of bile leakage (a patient who was kicked by a horse) was reported by Whipple in 1898 [5].The term “biloma” was coined by Gould and Patel in 1979. These authors reported a patient with extrahepatic bile leakage who had a history of upper abdominal trauma from a fight [6]. Spontaneous biloma is extremely rare, and choledocholithiasis and cholangiocarcinoma are reported as etiologic factors in most of these cases [3]. Rare causes have been reported, such as primary pancreatic malignancy [1], sickle cell disease [7,8] and gallbladder tuberculosis [9]. We emphasized that the spontaneous biloma was related with sclerosing cholangitis because there was no risk factor for developing a biloma in this case, including trauma, gallstone, abdominal intervention from biopsy or surgery, or malignancy in the CBD and/or periampullary region. It was not found any further reports about spontaneous biloma-related sclerosing cholangitis in the literature.
The mechanism of spontaneous biloma formation is still unclear. One currently suggested pathogenic mechanism is increased intraductal pressure due to obstructive lesions or infarctions of the bile ducts. Trivedi et al. reported the first patient with spontaneous biloma, which occurred secondary to pancreatic malignancy, in 2009 [1]. They commented that an acute elevation in biliary pressure is unusual because of the relatively slow onset of ductal obstruction that occurs with pancreatic neoplasms [1]. Middleton et al. reported that spontaneous biloma occurred as a possible sequel of hepatic infarction in a patient with sickle cell disease [8]. Our case had benign strictures in the CBD, however there was no cholestasis upon her first admission and cholestasis could not caused by medical treatment for sclerosing cholangitis in our opinion. So, we suggested that the development of spontaneous biloma was related with inflammation of the intrahepatic biliary ducts due to sclerosing cholangitis rather than to benign strictures. It may have caused the increased pressure in CBD, however we could not determine the source of the bile leak and the level of the increased biliary tree pressure.
Gallstones or extrahepatic biliary ductal compression, that was caused by a biloma must be kept in mind in the presence of increased levels of serum ALP, GGT, and total and direct bilirubin [10]. Antoine et al. reported the first postoperative biloma with extrinsic compression and obstruction of the CBD. Their case was the first with extrahepatic biliary compression secondary to biloma [10]. In our case, cholestasis developed on the tenth day of hospitalization (total bilirubin 5.7 mg/ dl, GGT 398 U/L), which was attributed to the extrinsic compression of the CBD by a huge biloma. It was confirmed that the proximal portion of the CBD could not be displayed, and there were no stones in the gallbladder and/or biliary tree on ultrasonography and MRCP images.
The clinical findings of spontaneous biloma usually consist of abdominal pain, distension, anorexia, nausea, chills and fever. The leucocyte count, ESR and CRP values may increase in the presence of concomitant cholangitis. The necessary imaging methods for the diagnosis of the biloma are ultrasonography, computed tomography, MRI and MRCP. ERCP can be used for both diagnostic and therapeutic purposes [11]. Our case presented with abdominal distention and pain, but her biloma was misdiagnosed as massive ascites on the first abdominal ultrasonography. The diagnosis of biloma was confirmed by subsequent abdominal ultrasonography, MRI and needle aspiration.
Percutaneous and endoscopic interventions are appropriate therapeutic approaches, and these methods are preferred over surgery as the first step in treatment [3,12,13]. Surgery should remain the last option because it is associated with numerous complications [14]. In our case, percutaneous catheter drainage was performed initiallybecause of the serious abdominal pain by capsular stretching. Thereafter, we performed an ERCP to reduce the intraductal pressure and to prevent the recurrence of biloma and cholestasis. ERCP showed the compression of CBD by a huge biloma. The bile drainage ceased and the cholestasis resolved completely after endoscopic sphincterotomy and placement of CBD stent. Three months later, the patient had no complaints, and no sign of biloma was seen on MRI.
4. Conclusion
To our best knowledge, this is the first report of spontaneous biloma associated with sclerosing cholangitis in the literature. We suggested that the spontaneous biloma was related with inflammation ofthe intrahepatic biliary ducts due to sclerosing cholangitis, rather than to benign strictures that was causing the increased pressure in the CBD. So as this may not always be accompanied by cholestasis initially. Percutaneous drainage and/or endoscopic biliary decompression are curable and precise therapeutic approaches.
Competing Interests
The authors declare that they have no competing interests.
Abbreviations
MRI: Magnetic resonance imaging
MRCP: Magnetic resonance cholangiopancreatography
CHD: Common hepatic duct
IBD: Intrahepatic bile ducts
CA 19-9: serum carbohydrate antigen 19-9
CEA: carcinoembryonic antigen
ERCP: Endoscopic retrograde cholangio-pancreatography
CBD: Common bile duct
TPN: Total parenteral nutrition