1. Introduction
Gastrointestinal stromal tumors are the most common mesenchymal tumor of the gastrointestinal tract. GIST presumed cell of origin is the interstitial cell of Cajal (ICC), a pacemaker cell that controls gastrointestinal (GI) tract peristalsis; however they can rarely occur in other intra abdominal tissues. Stomach is the most common location (40-70%), followed by small intestine (20-40%) and colorectum (5-15%) [1]. When they arise outside the gastrointestinal tract as primary tumor they are called extra-gastrointestinal stromal tumors (EGIST). The EGIST can be located in the omentum (greater or lesser), mesentery, liver, retroperitoneum, pancreas, etc [2].
GISTs are mainly sporadic and maybe associated with mutations [3]. More than 95% of GIST express the KIT protein (CD117), a transmembrane tyrosine kinase receptor for stem cell factor and recently DOG-1 has also been suggested as a useful diagnostic marker. KIT positive Cajal cells were not found in normal omental tissues failing to support the presence of these ancestral cells for GIST in the omentum. The molecular pathogenesis of GISTs is usually driven by activating mutations of the KIT gene that encodes the CD117 oncoprotein and platelet derived growth factor receptor-alpha (PDGFRA), which make these markers the most specific and sensitive for GIST [4,5].
What is known about their origin is less than little but as a common sense, their histological appearance and immunophenotype are identical to classical GIST. IT is thought that they represent either GISTs that have separated from GI tract wall, or independent growths of mesenchymal cells of the omentum and mesentery from which they originate. More data are needed to clarify.
Considering EGISTs we spilt them apart between solitary omental GISTs and multiple omental GIST. The former, whether attached to stomach or not, had a gastric GIST-like histology, mutation profile, and a low biologic profile with better survival rate and prognosis in majority of patients, even attached to stomach or not. On the other hand, multiple omental GIST had clinicopathologic features of metastatic or detached small intestinal GISTs harbor worse prognosis [6].
Depending on location and tumor size, clinical manifestations can occur but are very unspecific like abdominal pain (49%) or abdominal mass (20%). Tumor size and mitotic activity are considered to be the most useful parameters of metastatic risk stratification [7].
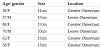
We report 4 cases of greater omental GIST in women and 2 cases of lesser omental GIST in men. The median age was 62 years and the range of tumor size was 11-20 cm. Them behavior was slight uncommon even with the rarity of studies available.
Our knowledge about EGISTs is based on accumulated data from individual case reports. In this paper, we describe some of our EGIST cases while a literature review is also made.
2. Results
Our data is quite reduced however we present clinicopathological features quite rare. There were 4 females and 2 males. The patient age ranged from 34-88 years (median 62 years). All of them complaint about abdominal mass with a slight abdominal discomfort. Only one patient referred abdominal intense pain. Four omental tumors were located in greater omentum, all in women and the other two were in men (table 1).
All patients were submitted to computer tomography (CT). A heterogeneous mass with low density and faith enhancement was observed in all patients. Abdominal angiography was performed in order to observed the tumor vascularity.
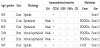
Greater omentum tumors ranged from 11-17 cm in maximum diameter and the lesser omentum tumors had 17 cm and 20 cm each. There was no adhesion to adjacent organs and structures but a pinpoint to the stomach. All the omental tumors were solitary. Enucleation of them was possible in all cases. Evidence of metastasis was not found in the abdominal cavity. Almost all were grossly well demarcated reddish-gray solid tumors, showed irregular modularity, contained focus of necrosis and cystic nodules. As shown in table 2, 4 were composed of proliferating epithelioid cells and myxoid cells with interlacting bundle pattern. The cellularity was relatively high and the frequency of mitotic figures was 2/50 high power fields (HPF).
C-Kit proto-oncogene protein product (CD117) positivity was detected in only one male, on the other hand, weakly positivity was detected in the rest cases, as well as positivity of neuron-specific enolase (NSE). Only CD34 was positive in all tumors. There was no staining for cytokeratin (CK), alpha-smooth muscle actin (SMA) or S-100 protein in any case.
Five patients carried the PDGFRA mutation and only one had not shown it (also negative for CD117- wild type). Direct sequencing demonstrated mutations in the PDGFRA gene exon 12, codon 561, encoding a thymine to adenine substitution. Three of the mutations occurred at exon 12 promoting Asp for Val substitution and one occurred in exon 18.
All were stratified as high-risk tumors. All of them underwent to complete removal with negative macroscopic and microscopic margins. Location was not an independent risk factor for prognosis. All of them undertook imatinib therapy with good results after 3 years of follow-up they are still disease-free.
3. Discussion
Clinicopathological features and prognosis of omental GISTs are limited due to the extremely rare incidence. Stromal tumors can occur anywhere these cells exist in the gastrointestinal tract, frequently in stomach and small intestine. EGISTs are rarely found in the peritoneum, mesentery and omentum.
Among available studies, the median age at diagnosis is about 60 years with a male female ratio 1:1. Miettinen, et al., reported that only 3% of GISTs are diagnosed before the age of 21 years and GISTs arise only rarely in children [8]. Clinical manifestations of GISTs are highly variable and it depends on tumor size and location. Omental GISTs can remain silent despite their size.
The most common complaint is an abdominal mass and/or abdominal pain, as our reports, but they are usually asymptomatic and found incidentally by imagological exams. Overall, almost 50% of GISTs have local or distant metastasis at the time of presentation [9].
Still nowadays, the precise etiology of omental GISTs remains unclear.
Having in mind the presence of ICC reported in many organs outside the GIT, it is rational to suppose that EGIST may derive from common precursor cells that differentiate into the ICC-derived neoplasm during their development. Another string advocated that tumor might come from pluripotent stem cells located outside the GIT. Another hypothesis is an extramural extension of a stromal tumor within the GIT. Sakurai et al demonstrated the existence of ICC-like cells focally in the omentum at 21 weeks of human gestation. However, it is unknown whether they develop in situ or migrate from the ICC of the tubular GI tract at particular point in fetal development [10,11]. They also reported in the normal omentum CD117+/CD34+ mesenchymal cells, like Cajal cells, from which the EGIST may theoretically arise.
Our reports are due to omental location. Feng, et al., reported that greater omentum was the most prevalent location, however another series from Fagkrezos, et al. and Dedemadi, et al., ascertained that there is no difference in incidence between lesser and greater omentum [11]. Our cases are insufficient to have the same conclusion.
Omental GISTs have been reported as solitary or multiple tumors. The largest study available belongs to Miettinen, et al. in which almost 50% of the solitary omental GISTs were attached to or involved the gastrointestinal tract, and the histologic features were similar to gastric or small intestinal GISTs. Considering this, they think that solitary type is actually externally extending gastric or small intestinal GISTs, and many others may have lost their original connection to the stomach or small intestine and become parasitically attached into the omentum [1,6]. They present a better median survival and better prognosis when compared to multiple omental GIST. A very few number of solitary EGISTs involved small intestine or showed small intestinal GIST morphology, which suggests that there is only an infrequent relationship between solitary omental and small intestinal GISTs.
In contrast, multiple omental GISTs had histological features of small intestinal GISTs. Usually they are advanced tumors that maybe metastases from an overlooked primary tumor, or have evolved from small intestine or stomach [1].
As said previously, in our cases, there was no adhesion to adjacent organs and structures but a pinpoint to the stomach. All the omental tumors were solitary. Evidence of metastasis was not found in the abdominal cavity.
Mutations in the KIT (exon 11) are the most common in GISTs of all sites accounting for almost 95% of GISTs and 40-50% of EGISTs. Spontaneous receptor dimerization and activation occurs when exon 11 is affected by KIT gene mutation [12,13]. Different mechanism results in uncontrolled KIT signaling if mutation occurs in exon 9 (specific for intestinal type), 13 or 17. A subset of GISTs which are negative for KIT gene mutations (2-5%) are positive for PDGFRA, which have been identified in exon 12, 14 and 18, nearly exclusively in gastric GISTs, almost in epithelioid type. About 10% are negative for both mutations, and we had one patient being both negative. PDGFRA mutations were chiefly detected in the solitary tumors, as we can see in our data, indicating similarity of the solitary tumors to gastric type GISTs, in which this mutation occurs [14,15].
Percentages may slight vary among studies, but most GISTs display spindle cell morphology (60-70%), whereas a minority is epithelioid (30-40) or mixed phenotypes (10%) [12]. As we far know, no correlation between prognosis and histologic type has been found. Spindle cell morphology correlates with KIT mutations and epithelioid type and mixed cell morphology correlates with PDGFRA mutations, both correlations were reported by Feng, et al. and Miettinen, et al. curiously, most of tumors in our patients had epithelioid and mixed phenotypes but we cannot make safe correlation based on our sample. Data indicated that KIT and PDGFRA mutant GISTs probably represent two distinct clinicopathological and molecular genetic disease entities. However, this needs further investigations in depth.
Antibodies to CD34 and CD117 appear in most GISTs. About 50- 80% of GISTs stains for the former while CD117 is expressed in 80- 100% of GISTs. CD117 was weakly positive in our report contradicting the majority of cases described, but CD34 stained in all. CD117 is not expressed in smooth muscle or neural tumors which helps in distinguish GISTs from another GI mesenchymal tumors [13,16].
It´s known that multiple omental GISTs had histological features of small intestinal GISTs. This relationship is supported by the occurrence of KIT exon 9 mutations characteristic of the small intestinal GISTs, and a lower frequency of CD34-expression, as seen in the small intestinal type [17]. Gastric type was the main type of our report. Nevertheless, they may show smooth muscle actin (SMA) positivity but are negative for desmin and S-100 protein. Omental tumors in our report were CK, SMA and S-100 negative.
The radiological features of omental GISTs without myogenic or neurogenic features have not been established. Generally, they may be similar to those of omental leiomyomas and leiomyosarcomas. Most GISTs with myogenic features are demonstrated as hypervascular tumors with clear margins on computer tomography (CT) and angiography. It was also doubtful for us make a diagnose based on both exams.
Computer tomography (CT) is the primary modality of choice for the diagnosis. Usually tumor size exceeds 10 cm, have irregular margins, heterogeneous and calcifications characteristics among others [18]. CT enterography uses large volumes of oral contrast which is more helpful than conventional CT [19].
Magnetic resonance (MRI) is more accurate than CT for delineating rectal GISTs. Esophagogastroduodenoscopy (EGD) show mainly sub epithelial lesions as a bulge with normal appearing mucosa. Studies show a very sensitive procedure but less specific for the location of sub epithelial lesions. Histologic confirmation is requested. Endoscopic ultrasound (EUS) is highly specific (100%) and sensitivity (92%) in differentiating submucosal tumor from extrinsic compression [20]. GISTs associated at high risk for malignancy depends on lesion size (more than 10 cm), cystic change and surface ulceration. From literature, tumor size exceeds 10 cm in majority of reports which classifies them as high-risk category. The only way to assess GIST malignant potential is surgical resection and histological analysis. Overall, studies performed by some authors concluded that EUSTrucut biopsy should be considered as an alternative to EUS-fineneedle aspiration (FNA) when technically feasible, because achieved a better diagnostic accuracy [21].
Positron emission tomography (PET)-CT using [18] F-fluorodeoxyglucose (FDG) detects cancer based on changes in tissue metabolism. It can be used for initial staging and to monitor disease progression and it´s been described 86% of sensitivity and 98% of positive predictive value in prediction of early response to therapy in recurrence or metastatic GISTs [22].
The Choi criteria is the most used in to achieved the tumor response to therapy, which is measured based on decreasing in tumor size by 10% on contrast enhanced CT [23].
Compared to a GIST tumor, EGIST harbors poor prognostic factors, including high proliferative indices, a larger tumor size, lymph node involvement, and distant metastasis. It can be attributed to the fact of development outside GIT may delay the presentation. There are several reports that tumor size does not impact the prognosis of EGIST patients and subsequent with patient survival. However, studies found that the mitotic rate showed a tendency to be associated with survival.
According the National Institutes of Health algorithm for assessing malignancy of classical GISTs, most omental EGISTs would be classified as high-risk due to their large size alone (>10cm). However, as we mentioned, the tumor size is not a reliable prognostic parameter EGISTs [24].
Surgery is the modality of choice for primary and localized GIST. For treatment, the main goal is to achieve complete resection with negative microscopic and macroscopic margins, function preservation while avoiding tumor rupture and injury to the pseudo capsule. Lymphadenectomy is not indicated as nodal metastasis is rare [25].
Imatinib is the most used drug for treatment as adjuvant therapy or neoadjuvant. Demetri, et al. evaluated the efficacy of imatinib on metastatic disease showing partial response or stable disease [26]. Heinrich, et al. has demonstrated that presence of KIT exon- 11 mutation had better outcome with imatinib comparing to KIT exon-9, however using the drug the made improves on response rate (complete/partial) [27].
Drugs like sunitinib had shown good results in patients whom were intolerant or resistant to imatinib, based on Demetri, et al. evaluation. Same authors evaluated the efficacy and safety of regorafenib after failure of treatment with imatinib or sunitinib with good results in patient’s survival [28].
Early detection and treatment of relapse are the main follow- up goals. Abdomen and pelvis CT are the modality of choice or MRI as alternative. For low risk patient annual CT over 5 years, in contrast, for high risk patients CT is recommend every 6 months. Evaluation should be appropriated for each patient, each tumor and each response as well.
From our study and with available data, we might think, what we know? GISTs tumors are mainly located ate stomach and small intestine and when they arise extra-gastrointestinal tract are called EGISTs. Majority of them have KIT and PDGFRA mutations almost in the same exons, however exceptions are reported. Globally, EGISTs have worse prognosis than GIST.
How can we deal with it? Considering the rarity of this tumors, a very few studies are available and we can only support our knowledge based on them and also case-reports. So, more data are need to understand it´s origin and behavior as well as the better treatment. Nevertheless, complete surgical removal of tumor when feasible and imatinib therapy when indicated are the main weapons for the patient, although the response is slightly worse that with GIST. New therapies are on the way but more studies are required.
4. Conclusion
The majority of omental GISTs occurred in greater omentum, exceeded 10 cm in diameter and were high risk. The incidence of epithelioid cell morphology and PDGFRA mutation were relatively high in omental GISTs. The histological type was correlated with location and mutational status. Mitotic index was risk factor for prognosis of omental GISTs. Omental GISTs differ significantly from gastric GISTs in respect to clinicopathologic features. The prognosis was comparable between omental and gastric GISTs.
EUS and improved knowledge of pathogenesis of GIST, accurate identification and differentiation of GISTs from other submucosal tumors are achieved. However, due to omental GISTs tumors rarity, they still layered with a great deal of ignorance regarding their immunobiological characteristics, their malignant potential and consequent prognosis, and so the guidelines applicable are the same for GISTs.
Surgery is still the best treatment but newer endoscopic techniques are on the field trying to gain some role.
Adjuvant therapy following resection of localized tumor with Imatinib has become a common standard of care mainly in highrisk tumors. It´s has been carried safely and may prevent relapse to prolong long-term survival. It is crucial to analyze accumulating data from case reports and series in order to achieve more depth and detailed understanding of primary omental GISTs.
Multidisciplinary approach is the most suitable treatment option for patient.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
NB and TL had contributed in designed and review of the manuscript; NG review of the manuscript; JK had contributed in patient observation and operation.