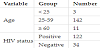1. Introduction
Cervical cancer (CC) is the fourth most common cause of cancer death in women worldwide [1]. A recent study reports a continuous increase in the incidence of CC in South African women from 1998 to 2012 [2]. The causative link between CC and Human Papilloma Virus (HPV) infection is well described, especially for high-risk oncogenic HPV types 16 and 18 [3]. The two primary oncogenes within the HPV genome, E6 and E7, are required for CC oncogenesis [4-6]. E6 and E7 drive oncogenesis following integration of the oncogenes into the host genome by deregulation of P53 and pRB (reviewed in [7]), loss of cell cycle control and subsequent malignant transformation. Co-infection of HPV with human immunodeficient virus (HIV) further increases the incidence of CC [8,9], with high-grade precancerous cervical lesions in HIV-seropositive patients and frequent involvement of the lower genital tract [10]. HIV-seropositive patients develop precancerous cervical lesions [11-12] and invasive CC almost ten years earlier than HIV-seronegative patients on average [13], which is often concomitant with poor therapeutic response.
CC preventive approaches are well-established worldwide; however, screening strategies and health policies vary greatly across different countries. South African methods and intervals between Pap smear tests are not uniform between public and private sectors, which coincide with low- and high-resource areas, respectively [14]. The South African Advisory Board advices HPV-based screening methods over cytology because of their higher sensitivity, which allows for a longer and safe screening interval [14]. However, cytology is still commonly used, especially in the public sector. Following positive cytologic and/or HPV screening tests, patients frequently undergo colposcopy with biopsy or therapeutic excision of precancerous cervical lesions using Large Loop Excision of the Transformation Zone (LLETZ) procedure [15]. The histological evaluation of biopsies collected by LLETZ is diagnostically advantageous by determining whether the precancerous cervical lesion was excised completely, incompletely or inconclusively.
The treatment success or failure of LLETZ procedures vary between studies, resulting in a high degree of uncertainty and inconsistency in the follow-up management of women with precancerous cervical lesions. This study addresses this gap in knowledge by determining the accuracy of using LLETZ biopsy margin status to predict followup pap-smear results. To that end, Positive Predictive and Negative Predictive Values of LLETZ biopsy margin status were calculated and used to define treatment failure and success. This study contributes to the establishment of more effective follow-up strategies and the optimization of testing intervals for the management of precancerous cervical lesions. A long-term goal in the field is to decrease cervical cancer morbidity and mortality in developing countries. A secondary objective of this study was to evaluate the predictive value of papsmear for low- and high-grade precancerous cervical lesions in predicting LLETZ biopsy histology.
2. Methods
2.1 Patients and data collection
A retrospective cohort study was completed using data collected from 156 patients enrolled in this study in 2016 at the Ladysmith provincial Hospital Colposcopy clinic. Patients included were women who presented for the first time at Ladysmith provincial Hospital Colposcopy clinic in Ladysmith between 01 April 2016 and 31 December 2016. Names and hospital numbers of all women who were evaluated at the colposcopy clinic during the study period were retrieved from the colposcopy clinic admission book. The files containing selected names and hospital numbers were retrieved from hospital records, and the information needed was retrieved from the hospital files and hospital site National Health Laboratory Service. All patients underwent initial Pap-smear testing and came to the hospital colposcopy clinic because of abnormal results. Patients were included in the study if they presented at colposcopy clinic for the first time with cytology results showing precancerous squamous cell cervical lesions and had HIV status results. Data included age, HIV status, precolposcopy and LLETZ Pap-smear results, LLETZ biopsy histology results including margins status and post-colposcopy and LLETZ Pap-smear results. Cytologic and histologic samples were evaluated following the Bethesda System for low- (CIN I) and high-grade (CIN II/III) lesions.
2.2 Ethical approval
The protocols for data collection and analysis were approved by the KwaZulu-Natal Department of Health Ethics Committee (Ref # KZ_202006_013) and Umgungundlovu Health Ethics Research Board (Ref # UHERB 006/2019).
2.3 Data analysis
Calculations for predictive values and 95% confidence intervals (CIs) were calculated using R with the epiR package [16]. The 95% Confidence interval for HIV infection rate was calculated using a two sided binomial test.
3. Results
3.1 Patient demographics and eligibility
Patients were between 23 and 86 years of age. HIV positivity was found in 122 of 156 patients, 78% (CI 95%: 70.9% 84,4%), and 34 of 156, 22% (CI 95%: 15.5%, 29.1%) were HIV negative. The percentage of HIV positive patients stratified by age was 100% (3/3) for those under 25 years of age, 80% (113/142) for those between 25 and 59 years and 55% (6/11) for patients 60 years of age and older. (Table 1 and Figure 1).
3.2 Pap-smear PPV for HSIL and LSIL
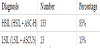
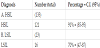
Of the 156 patients who were referred with abnormal Pap-smear results, 133 (98 HSIL + 35 ASH-C, 85%) were diagnosed with High- Grade Squamous Intraepithelial Lesions (HSIL) and 23 (14 LSIL + 9 ASCUS, 15%) with Low-Grade Squamous Intraepithelial Lesion (LSIL) (Table 2). Among biopsies collected by LLETZ procedure and evaluated by histology, 121/133 (91%, CI95%: 85%–95%) of highgrade lesions were confirmed to have CIN 2+ lesions, and 16/23 (70%, CI 95%: 47%–87%) of low-grade lesions were confirmed to have CIN 1 (Table 3).
3.3 LLETZ biopsy margin status PPV (treatment failure) and NPV (treatment success)
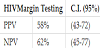
Between 3 and 4 months post-colposcopy and LLETZ 125/156 (80%) of the patients returned to the clinic for post-LLETZ Pap-smear testing, and 20% (31/156) of them defaulted. Among the total of 125 compliant patients, the LLETZ procedure margin status identified 39/125 (31%) of them with negative margins, 48/125 (38%) with positive margins and 38/125 (30%) with inconclusive margin status. Among patients that were diagnosed with negative margins, 24/39 (62%) were confirmed to have normal Pap-smear (treatment success), while 15/39 (38%) had abnormal Pap-smear (treatment failure). Of the 48 patients diagnosed with positive margins, 28/48 (58%) were confirmed to have abnormal Pap-smear (treatment failure), while 20/48 (42%) were found to have normal Pap-smear (treatment success). Of those with inconclusive margin status, 58% were confirmed to have normal pap-smear (treatment success), and 42% had abnormal Pap-smear (treatment failure) on follow-up (Figure 2). These results translate into a PPV of 58% (CI95%: 43%–72%) and an NPV of 62% (CI95%: 45%–77%) for LLETZ biopsy margin status prediction of follow-up Pap-smear results, indicating treatment failure and success, respectively (Table 4).
3. Results
HSIL is the classification of precancerous cervical lesion that is most relevant to the development of cervical cancer. Therefore, the PPV of a screening test for identification of HSIL is most relevant to identifying risk of cervical cancer. In the current study, this predictive value was 91% for Pap-smear screening in the setting of a South African hospital. This value indicates reliable identification of highgrade lesions and prediction of risk of cervical cancer. Other studies in low-resource areas, including a cross sectional analysis of 1034 women in Vietnam, have found visual inspection with acetic acid to be sensitive for screening and identification of HSIL (CIN2+) with a PPV of 51.2%, but less precision was indicated with Pap-smear at a PPV of only 83.3% [17]. Another study in western Kenya showed a PPV of only 39.7% for Pap-smear [18]. In some very low-resource areas, visual inspection with acetic acid may out-perform other screening methods and be more accessible. Differences between these findings and current results may be due to the specific single-center setting evaluated here, although the setting of the current study is representative of South African heath care settings. In comparison, studies in developed countries, including a multi-center study in France, have shown a PPV of conventional Pap-smear for detecting HSIL of 90%, which is similar to that found in the current study in South Africa [19]. Inconsistency in the predictive value of Papsmear between areas is a subject that justifies further comparative investigation. Pap-smear is a relatively low-cost option that may be preferable in low- to mid-income countries where punch biopsy, loop excision and HPV testing may be less accessible because of cost. These factors together with the findings of a Pap-smear positive predictive value for HSIL that is comparable to LLETZ biopsy histology indicate that Pap-smear may be the best screening option for such countries. The current results support the use of Pap-smear as a primary screening method in South Africa and in middle- and low-income settings if they have availability and demonstrable proficiency. Given variability between settings, demonstration of precision and sensitivity of testing within particular settings is of importance.
Pathologic review of surgical margins is used following LLETZ biopsy to determine the presence of residual cervical lesions. In the current study, the precision of LLETZ biopsy margin status for prediction of treatment failure (PPV) and treatment success (NPV) in a South African hospital setting were evaluated. PPV and NPV of margin status were determined to be 58% and 62%, respectively, reflecting modest predictive values for residual disease. Another recent study in Finland found margin status to carry a PPV of only 8% but a NPV of 99% [20], suggesting variability in precision between settings, which may be due to pathologist experience, surgical technique or sampling differences. It has been suggested that thermal destruction of the surgical margin limits the interpretability of LLETZ biopsies and therefore, limits the diagnostic and therapeutic value of this method [21]. Testing for high-risk HPV following surgical excision is considered to be sensitive and more accurate than cytology or margin status [22] and should be included in follow-up when available and accessible.
While treatment success rates for LLETZ are reasonably high in South Africa with 17% having residual disease after 4 months, default rates are also high (81% in one study) [23]. Even the 20% default rate in the present study presents a problem for appropriate disease management following LLETZ procedure. Conversely, a study in the UK had a default rate of only 7% among 3426 women [24]. Default rates for follow-up post LLETZ with an HSIL diagnosis vary widely between different parts of the world, highlighting a need to develop effective tracing strategies to mitigate lack of follow-up among women presenting at colposcopy clinics. Examples of such strategies include pre-procedure counseling regarding the risks of defaulting on followup, telephone or email follow-ups and home visits by local health care workers or referring clinics.
In the cohort included in the current study, 78% were positive for infection with HIV, highlighting the prevalence of co-infection with HIV and HPV in South Africa. Similar rates of co-infection were found in cohorts of HIV positive men and women (76.6% and 74%, respectively) in previous studies in South Africa [25]. Among HIV positive women over 30 years of age referred for colposcopy in Cape Town, South Africa, 70.2% were found to have high-grade cervical dysplasia [26]. The highest prevalence of HPV infection worldwide is found in areas with high HIV prevalence, such as sub-Saharan Africa, according to the WHO [27]. Cervical intraepithelial neoplasia is indeed more prevalent in South African women that are HIV-positive compared to those who are HIV-negative [28]. These epidemiologic considerations contribute to the importance of appropriate cervical cancer screening in South Africa, especially among HIV-positive women.
There are certain limitations to this study. The retrospective; singlecentered nature and modest sample size limit the ability to generalize findings and the power of the study. And since the current study does not cross public and private sectors or low- and high-resource areas, disparities between these settings cannot be addressed.
4. Conclusion
Pap-smear testing had reasonably high PPV (91%) for HSIL to be effectively used as a low-cost cervical cancer screening method in a middle- to low-income hospital settings. However, the PPV (58%) and NPV (62%) of LLETZ biopsy margin status are modest, justifying a careful three to four months follow-up after LLETZ procedure. A 20% follow-up default rate after LLETZ procedure in this cohort suggests that robust strategies to improve follow-up compliance need to be developed.
Competing Interests
The authors declare that they have no competing interests.