1. Introduction
Granulosa cell tumor of the ovary (GCT) accounts for 2% to 5% of all ovarian cancers and can be divided into two subtypes according to the differences of the age of patients [1,2]. Rokitansky (1859) was the first to describe granulosa cell tumor of the ovary as a single tumor entity [8,12,13]. About 95% of GCT belong to the adult granulosa cell tumors (AGCTs), and others are juvenile granulosa cell tumors (JGCTs) [1]. Granulosa cell tumors can occur at any age, but are more common after menopause, with a peak incidence between 50 and 55 years of age [13]. The main differences between GCT and other ovarian carcinomas are that GCT can lead to abnormally secreted hormones (estrogen, inhibin, and Müllerian inhibitor), which can cause hormonal imbalances [1]. JGCT occurs only before puberty and may manifest in the form of hyperestrogenism [1,3,4]. Clinical features of AGCT include abnormal uter ine bleeding in postmenopausal patients and menometrorrhagia in youngers [1]. A continuous growth of the tumor can cause in other cases nonspecific, abdominal symptoms [13].
The main risk factors of GCT include nulliparity, fatness, oral contraceptives and family cancer history [1].
2. Pathomorphological Features of the Granulosa Cell Tumor
The adult type has various growth patterns: microfollicular (characteristic, with Call-Exner body), macrofollicular, trabecular, insular, solid-tubular, gyriform and diffuse (sarcomatoid) [7]. The juvenile type has solid and follicular structures [7]. The neoplastic granulosa cells of the adult type are irregularly arranged, cytoplasm poor and contain pale nuclei, often with longitudinal groove ("coffee bean kernels") [7]. In the juvenile type, however, there are broad cytoplasmic cells with eosinophilic cytoplasm and polymorphic nuclei without longitudinal groove [7]. The number of mitoses in the adult type usually does not exceed 2/10 HPF, while it may be significantly higher in the juvenile type and also atypical mitoses occur [7]. Molecular genetically, a somatic missense mutation in the Forkhead Box L2 gene (FOXL2) (C134W) is present only in adult GCT patients and does not occur in other ovarian carcinomas [22]. FOXL2 is a transcription factor that plays a crucial role in ovarian function, especially in the proliferation and differentiation of granulosa cells [22].
3. Case Report
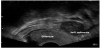
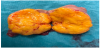
A 55-year-old postmenopausal woman (gravida 3, para 3) suffered from abdominal pain. A year ago, a fractionated abrasio uteri was performed because of a postmenopausal bleeding. Histological examination revealed no pathological findings. After a pelvic examination, the transvaginal ultrasound revealed a mass measuring 4.5cm x 4.0 cm in the left adnexal region (Figure 1). Adnectomy of the left side was performed via laparoscopy (Figure 2). The patient at that time refused adnexectomy on the opposite side. The histopathological results showed a cellular, mitoticallyactive fibroid and the cells express the smooth muscle marker actin, as well as inhibin-alpha, calretinin, CD99 and CD56 in negativity for desmin, estrogen receptor, pancytokeratin, CD10, WT1, CK7, EMA and S100. The proliferation rate measured with Ki67 is about 2-5%. Based on the differential diagnoses to be considered and the possible therapeutic consequences associated therewith, a molecular genetic testing of the preparation was performed.
Molecular genetically, a somatic mutation p.C134W was detected in the FOXL2 hotspot codon 134. The hotspot regions in the DICER1 exons 24 and 25 show no mutation. Thus, morphological diagnosis of an adult granulosa cell tumor is supported by detection of the typical mutation in the FOXL2 gene. Based on the initial histological findings further was performed laparoscopy with hysterectomy and right adnexectomy. The Uterus with adnexa right was extracted vaginally as a whole. The Douglas cytology was inconspicuous, no evidence of malignant cells in the Douglas area. The results from both surgeries revealed a tumor with FIGO stage IA, and R0-resection was achieved.
4. Discussion
Granulosa cell tumor (GCT) is a malignant tumor originating from the sex-cord stromal cells of the ovary and represents 2% to 5% of all primary ovarian cancers [6,11]. Approximately 5% occur before puberty (juvenile GCT) and the majority (95%) of the cases are the adult type of GCT (occur in people of reproductive age and postmenopausal age) [1,6].
Hormonal secretion is characteristic of these tumors and can lead to meno- and metrorrhagia, postmenopausal bleeding or amenorrhea [13].
An adult granulosa cell tumor from other ovarian tumors is very diffcult to differentiate by conventional histological examination. In this regard, an immunohistochemistry and molecular genetic testing is necessary. Immunohistochemistry characteristic here is the positivity for inhibin-α, calretinin and CD99 and a negativity for EMA. 95-97% of adult granulosa cell tumors carry a unique somatic mutation in the FOXL2-C134W gene [22].
The detection of a mutation in the FOXL2-C134W gene leads to confirmation of the diagnosis of the adult granulosa cell tumor [9,22].
Complete surgical resection of the tumor is the mainstream of treatment [13]. The traditional treatment modalities followed are complete surgical excision of tumor with unilateral salphingoopharectomy in patients desirous of preserving fertility [8]. Total hysterectomy with bilateral salphingo- opharectomy in patients with completed family [8]. It remains to be clarified whether patients with stage I disease benefit from more extensive surgical treatment including total hysterectomy and bilateral salpingo-ophorectomy [13]. Repeated debulking procedures can contribute to mediumand long-term survival with good symptom control in patient with recurrent disease in selected cases [13]. Reccurence is predominantly located intraperitoneally [13]. To date, an adjuvant chemotherapie for patients with completely (macroscopic) removed granulosa cell tumor of the ovary is not obligatory [5,13].
Adjuvant therapy for advanced and recurrent disease has been discussed in various studies, the feasibility of different platinumcontaining chemotherapeutic regimen [13,14] has been demonstrated in prospective studies [13,15] and radiotherapy may also represent a treatment option for selected cases [13,16]. Some authors recommend polychemotherapy containing cisplatinum, vinblastine and bleomycin for patients with advanced stage or relapse [13]. Complete tumor resection should always be attempted, since residual tumor disease is associated with a poor prognosis [13]. In a multi-institutional study of 65 patients with granulosa cell tumor, Sehouli et al. showed that tumor residual after primary surgery was associated with a worse prognosis (p<0.001) [13]. The study showed no significant difference between the survival rates of patients with tumor rupture during the surgery (p = 0.25) [13].
The most important prognostic factor in this tumor types is the tumor stage [7].
The majority of patients are diagnosed in early stages of disease and overall prognosis is favorable [13]. Approximately 75% of granulosa cell tumors are diagnosed in stage Ia-c (FIGO), 20% represent stage II, 8% stage III and 6% stage IV of tumor disease [13,17,18]. The prognosis is significantly poorer for patients with advanced tumors with 10-year survival rates of 20% and in stage III and IV, respectively, similar to ovarian cancer [13]. 10-year survival rates are approximately 70-95% for stage I, 55-75% for stage II and 25% -50% for stage III [13,17-21].
Granulosa cell tumors are well known for late recurrence [13]. Hines et al. reported a patient with recurrent disease 37 years after initial diagnosis [13,23]. As a clinical consequence, lifelong followup for all patients with granulosa cell tumors is warranted and should not end after five years [13].
Competing Interests
The authors declare that they have no competing interests.