1. Background
Saliva plays an important role in maintaining oral health [1] . The constitution and quantity of saliva are main factors in oral health. These factors can be altered by different etiological factors such as diet, tobacco, alcohol, emotional state, ambient temperature and air humidity that could lead to hyposalivation [2] . Hyposalivationis associated with an occurrence of fungal infections, mucositis, dysphagia, caries lesion, instability of removable prosthesis, halitosis, dysgeusia and a decreased pleasure of eating [3] .
Smoking tobacco is one of the risk factors for decreased salivary flow rate. Saliva is the first biological fluid exposed to tobacco which contains many toxic substances, responsible for structural and functional changes in saliva [4,5]. Cigarette smoke has a large number of toxic components and is a significant source of oxidative stress through increased production of reactive oxygen species that can lead to intra and extra cellular changes and disturbances of salivary function [6,7].
As tobacco has toxic substances that can promote changes in the secretion (composition and quantity) and function of saliva, it is important to study possible salivary stimulants. The rhizome Zingiber officinale Roscoe, known as ginger, has been used as a spice for over 2000 years. The prokinetic activity of ginger was confirmed by Ghayur & Gilani [8] and by Ghayur, Khan, & Gilani [9] . Authors suggest that the effect of ginger can be attributed to cholinergic agonistic action on the post-synaptic M3 receptors [8,9]. Muscarinic M3 receptors are likely to be the principal mediators of cholinergic responses in salivary glands on the increase of salivary flow [10] . In which concerns the ginger eventual adverse effects, ginger is considered GRAS (Generally Recognized As Safe) by US Food and Drug Administration (FDA) and according with Kumar et al., a dose of 0.5-1.0 g of ginger powder ingested 2-3 times for periods ranging from 3 months to 2.5 years did not cause any adverse effects [11,12].
Regarding the ginger potential effect in the salivary flow and the safety maximum dose of 2-3g/day for 2.5 years, the aim of this study was to investigate the effect of a ginger infusion (0.5 g/250 mL) three times a day for a short period of 28 days, in the salivary flow of smokers with reduced salivary flow rate.
2. Materials and Methods
2.1 Subjects
After local recruitment advertisement (Torres Vedras, Portugal), sixteen smokers with reduced salivary flow ratendate above 20 years were selected for this study. The inclusion criteria were: smokers for more than five years, decreased non-stimulated (Qns) and stimulated (Qss) salivary flow rate (Qns flow rate ≤ 0.1 mL/min and Qss flow rate ≤ 0.7 mL/min) and age over 20 years. The exclusion criteria were normal salivary flow rate, allergy to ginger, pregnancy condition and regular drug therapy.
2.2 Ethical approval
This study was approved by the Portuguese state recognized Ethics Committee (Ethics Committee of Egas Moniz - Cooperativa de Ensino Superior, C.R.L.) and was carried out in accordance with the Helsinki Declaration of 1975 as revised in 2013. A written informed consent was obtained from each participant after given oral information about the study.
2.3 Study Design
The auto-controlled clinical trial consisted in the ingestion of a 250 mL ginger infusion with 0.5 g, three times a day for 28 days. Participants were selected after sialometry result, according to the inclusion criteria. After selection, data about age, gender and smoking habits (duration and cigarette number) was collected. The study was conducted throughout two evaluation moments. The first evaluation occurred before the beginning of the ginger ingestion period. Subjects ingested a ginger powder infusion (for three times a day/28 days ingestion). An explanatory document of the entire infusion preparation procedure was given to participants. The second evaluation occurred after the 28 days period. The compliance of the study protocol was verified and new sialometry was performed.
2.4 Saliva collection
Saliva collection was performed between 9 am to 11 am to avoid diurnal variation according with sialometry protocol [13] . Each subject was requested not to eat, drink, perform oral hygiene or smoke 60 minutes before and during the collection of saliva [13,14]. The subjects were seated and asked to spit in a saliva collecting container for five minutes. First, the amount of unstimulated saliva was measured during 5 minutes followed by the stimulated saliva collection measured for 5 minutes as well. During saliva collection patients were instructed not to swallow. After collection, the salivary flowrate was measured and expressed in mL/minute.
2.5 Ginger infusion preparation
The ginger (ginger powder, Biodharma, origin: India) delivered to the subjects was weighed, distributed in a sealed packet with 0.5g ginger powder each and given to each subject on the day of the first evaluation. All participants were informed about the preparation of the infusion: boil 250 mL of water (250 mL for one serving size); add ginger powder (one packet - 0.5g) to the boiled water and stir; leave it to stand for 5-10 minutes until it reaches room temperature. Participants were asked to mouthwash before infusion ingestion.
2.6 Ginger infusion analysis of phenolic content and antioxidant activity
The chemical analysis was performed in the used ginger infusion. Homogeneous samples were obtained and subjected to chemical analysis.
All reagents were pro analysis grade. The total phenolic content of the extracts was determined according to the Folin-Ciocalteu method employing gallic acid purchased in Acros Organics (Portugal), as standard [15] . The results were expressed as mg of gallic acid equivalent (GAE)/L of infusion. The absorbance was measured at 765 nm using a spectrophotometer (Perkin-Elmer Lambda 25).
The free radical scavenging capacity was studied using the ABTS (2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid, Sigma- Aldrich) radical assay, the antioxidant activity was measured as the ability of test compounds to decrease the color reacting directly with the ABTS•+ radical (an intensely colored radical cation monitored in the range of 600-750 nm) [16] . This assay was performed by TEAC (total equivalent antioxidant activity) using Trolox (6-hydroxy- 2,5,7,8-tetramethilchromane-2-carboxylic acid, Sigma-Aldrich) as standard (μmolTrolox/L) [17] . This test was performed for several extract concentrations in order to calculate IC50 (value defined as the total phenolic concentration required to achieve half maximal inhibition of ABTS•+ radical).
The inhibition of superoxide anion (O2•−) test was performed using NADH (Sigma-Aldrich), nitroblue tetrazolium (NBT, Sigma- Aldrich), Tris-HCl (Sigma-Aldrich) and phenazine methosulfate (PMS, Sigma-Aldrich). Absorbance was measured at 560 nm [16,17]. The inhibition percentage of the O2•− was calculated through equation (1).
2.7 Statistical Analysis
Statistical data analysis was performed using IBM SPSS Statistics version 24.0 for Windows (Armonk, NY: IBM Corp.). Data are presented as mean ± standard deviation (SD) or standard error of mean (SEM). The paired samples t-Student’s test was used to assess the difference between salivary flow rate before and after the ingestion of ginger infusion and Spearman's correlation coefficient was also used to correlate age and smoking habits (duration and cigarette number) with salivary flow rate (Qns and Qss). In the comparative statistical inference analysis, the level of significance was set at 5%.
3. Results
The participants had a mean age of 36 (±11) years, with a range from 24 and 58 years (Table 1). Among the sixteen subjects, nine (56%) were female and seven (44%) were male.
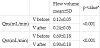
Results demonstrate a difference between the average of salivary flow rate before and after the ingestion of ginger infusion (Table 2). A statistically significant increase was observed in non-stimulated (Qns) and stimulated (Qss) salivary flow rate after 28 days (p < 0.001, paired samples t-Student’s test).
The correlation between the amount of saliva and age and smoking duration and number of cigarettes smoked per day was also explored. The results revealed a negative correlation between Qns and age (ρ = −0.67), with the amount of unstimulated saliva being significantly reduced (p = 0.003) with age increase. Also the results of the correlation of the duration of smoking and the amount of saliva (Qns and Qss) showed a, statistically significant, negative correlation (Qns, ρ= −0.71, p=0.001; Qss, ρ = −0.61, p= 0.010). The amount of unstimulated and stimulated saliva decreases significantly with the increase of smoking duration. However, the results revealed a weak, non-significant, correlation between the amount of saliva and the number of cigarettes smoked per day (Qns, ρ = −0.33; Qss, ρ= −0.22).
Concerning the chemical data, water infusion of Zingiber officinale Roscoe has 2.4 times more capacity of free radical scavenging compared with antioxidant standard Trolox through ABTS assay and TEAC (IC50) and revealed a high antioxidant activity by inhibiting the superoxide anion (Table 3).
4. Discussion
The results of the present study revealed a statistically significant increase in non-stimulated (Qns) and stimulated (Qss) salivary flow rate after 28 days of the ingestion of a 250 mL ginger infusion with 0.5g of ginger powder. These results are in agreement with previous clinical trial on type 2 diabetes mellitus adults, which demonstrated that the mean amount of saliva after using the ginger plant spray increased significantly. In addition, this study revealed that ginger herbal spray improved dry mouth on patients [20] .
Reduced salivary flow may have repercussions in the oral cavity and could alter the quality of life of the subjects [21] . The results of the present study demonstrate a decreased salivary flow rate in Qns and Qss with the increasing of duration of smoking. These data are in accordance with Gopal et al. [5] and Petrušić et al. [22] which demonstrated that long-term smoking is one of the factors that reduce salivary flow rate. However, the amount of saliva is not significantly associated with the number of cigarettes smoked per day. This result is in agreement with the study of Petrušić et al. [22] .
Cigarette smoke is a source of reactive oxygen species like the superoxide anion (O2•-), that can cause significant biological damage [23,24]. The increase of production of these species associated to smoking may exceed the capacity of the antioxidant defense system and result in stress and oxidative damage [25] . If the antioxidant action decreases, the free radicals are not eliminated and will be available to participate in harmful actions to the DNA (deoxyribonucleic acid) and may affect the secretory function of the salivary glands [26] .
The chemical analysis results are in agreement with Sabli et al. [27] and Tohma et al. [28] studies, where the used ginger infusion revealed a high a TFC and antioxidant activity (through inhibition of free radical ABTS•+ and the Osub2•−). These chemical properties could be associated with the increased flow rate observed in this study, as proposed by Gayur and kumar [9,11].
Besides the harmful effects of cigarette smoke, the decrease in salivary flow rate could be related to increased age, that may lead to changes in the composition and function of glandular tissues [26] . The meta-analysis performed in 2015 by Affoo et al. [29] suggested that salivary flow rate decreases with aging. The results of the present study demonstrated a statistically significant decrease of Qns with the increase of the subjects’ age.
5. Conclusion
In this study ginger infusion (0.5 g/250 mL three times a day) reveals to be a salivary stimulant and a high antioxidant activity which may be associated with the increase of the salivary flow rate found. These results suggest that use of antioxidant salivary stimulants such as ginger infusion may prevent salivary decrease.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.