1. Introduction
1.1 Background
Tooth extraction results in resorptive changes of the alveolar ridge [1]. In particular, there will be a significant loss of bone volume on the buccal aspect of the empty socket [2]. The bone loss after extraction reduces ridge width (horizontal reduction) by an average of 3.8 mm and ridge height (vertical reduction) by an average of 1.2 mm in the 18 first six months [2-4]. This loss of bone width and height may compromise the esthetic 19 appearance [5].
To prevent bone loss, an attempt can be made to influence bone and soft-tissue healing by taking appropriate measures following tooth extraction. The idea is to reduce the resorptive processes during socket remodeling. Various methods for stabilizing the bone after extraction have been described [6].
Incorporating different types of bone replacement materials or sealing of the empty socket with a membrane [7,8] or by plastic coverage using an advancement flap or a gingival graft [9] has been proposed and shown to have significant positive effects on bone loss [10,11]. Preserving the bone as much as possible is important for the stability, prosthetically correct position, and long-term functional and esthetic success of the implant treatment. If necessary, any advanced resorption of the bone must be compensated for by augmentation procedures, which are associated with significant cost and may potentially cause considerable discomfort to the patient.
Previous studies have shown that alveolar ridge preservation (ARP) reduces the extent of bone augmentation needed prior to implant placement [12]. However, the results were highly inhomogeneous. The nature and extent of the necessary augmentation procedures are not usually described in detail.
Various clinical or radiographic methods of measurement have been used to evaluate the success of ARP in clinical trials [13-15]. Measurements with a millimeter-scaled periodontal probe are strictly a clinical method for the two-dimensional determination of horizontal and vertical bone loss. For example, the buccooral width and the height are measured in two dimensions, based on predefined distances at the time of tooth extraction. On the day of reentry for implant placement, these distances are again measured and the results compared [13,16-20]. Measurements on models obtained by taking impressions at the time of tooth extraction and at the time of implant placement have been described as an alternative to intraoperative measurements [21].
Other authors performed measurements on models obtained before and three months after tooth extraction to identify the amount of bone resorption [22]. Dimensional changes of the bone have also been evaluated by radiological methods such as standardized intraoral periapical radiographs [23,24]. These radiographs were taken before the extraction, after ARP treatment, and at four months. Linear measurements were made at defined distances, and quantitative digital subtraction radiography showing the radiological changes of the hard tissue is additionally applied. Furthermore, three-dimensional radiological measurements based on CBCT data have been described. In one study, one CBCT was taken immediately after tooth removal, a second one after implant placement, and the third one at two years [25]. Evaluation was performed by measuring predefined distances on the CBCT images.
Besides the metric analyses of the bone level before and after ARP, changes in bone mineral density have also been measured [26]. This type of measurement again requires taking a radiograph immediately after tooth extraction and one at the time of implant placement. However, CT scans must be made to determine the bone density, as reduced radiation CBCT can provide only limited density information [27]. Virtual overlays of CBCT images have been described as an alternative way of obtaining data from evaluations and measurements of three-dimensional radiographs [28]. Here, CBCT images taken immediately after extraction and CBCT images taken five to six months later (for implant planning) [29] are superimposed.
Surface data can be derived from DICOM records using appropriate software programs. Thus, another study generated surface data from images obtained directly after tooth extraction and at eight weeks. These surfaces were once again superimposed based on nearinvariable anatomical structures, and the distances between the surfaces were graphically represented and measured [14].
We suspect that the less pronounced volume loss achieved with ARP will reduce the 4 surgical complexity of the subsequent implant treatment by minimizing the need for 5 augmentation procedures, and consequently lower the cost. The present paper aims to 6 introduce the study design, to describe the new, non-invasive measurement method, and 7 to present and discuss preliminary results from a pre-trial.
1.2 Objectives
This study tests the hypothesis that the use of a combination material consisting a collagen plug and a collagen membrane (1) reduces bone resorption after tooth extraction; (2) results in better preservation of the alveolar ridge (soft tissue and bone); (3) reduces the need for bone augmentation procedures in the subsequent implant treatment; and we want to examine whether (4) ARP is more cost-effective than the standard therapy with unassisted socket healing. Secondary hypotheses are that (1) ARP has an influence on the survival rate of implants; (2) ARP has an influence on the esthetic result of the implant-supported restoration; and (3) ARP leads to modified results on a histomorphometric level.
2. Materials and Methods
This study was designed as a single-blinded, randomized, controlled clinical monocentric trial. It will be reported according to the CONSORT statement [30-32]. Guidelines followed in the study design:
- World Medical Association Declaration of Helsinki (DoH) 1
- Clinical Investigation of Medical Devices for Human Subjects - Good Clinical 2 Practice (ISO 14155:2011) 3
- Guidelines of Good Clinical Practice (2001/20/EC)
The Ethics Commission of the Medical Faculty of the University of Ulm approved the 5 study design (Docket Nos. 337/12 and 41/14).
2.1 Trial design
The controlled clinical trial was designed as a single-center prospective, single-blinded, randomized, parallel-group study. The examinations were investigator-initiated. The study protocol is implemented unchanged.
2.2 Participants
The study participants are 88 patients in whom at least one maxillary tooth needs to be extracted and who desire replacement of the extracted tooth with an implant and a fixed prosthetic restoration. Such an extraction could be indicated for periodontal reasons or because of advanced tooth destruction by caries or trauma, precluding preservation of the tooth. A prerequisite for inclusion in the study is that a tooth or an existing implant is present immediately adjacent to the tooth to be extracted.
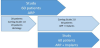
The participants of the pre-study and the main trial are divided into 3 subgroups: ARP was performed similarly in 44 patients. A histological examination was conducted in 20 patients; 8 patients had to await the product launch and testing of a new implant insertion tool; and in 60 patients, implant placement was carried using with the modified implant drill system and followed up appropriately (Figure 1).
Exclusion criteria:
- Age under 18 or legal incompetence
- Recognizable additional need for primary augmentation, such as an external sinus lift or extensive augmentation procedures in the immediate vicinity of the tooth to be extracted (in the case of multiple implantations)
- An intraoral situation that does not permit the insertion of the implant with the 6 aid of a drilling template (insufficient mouthopening capacity) 7
- Heavy smoking (more than 10 cig/d) 8
- Active periodontal disease 9
- Use of bisphosphonates 10
- Pregnancy 11
- Alcohol or drug abuse 12
- Chronic infectious diseases such as hepatitis or HIV 13
- Severe uncontrolled diabetes; the HbA1c long-term blood glucose indicator must 14 be less than 6.7%
The patient-specific exclusion criteria are regularly evaluated over the study period and 16 changes documented.
2.2 Settings and locations where the data will be collected
The study venue is a dentist’s office in Hilzingen (Germany). All patients are treated exclusively by one dentist (SiS). All patients are recruited from the patient base of the dental surgery. All patients have been informed about the study project verbally and in writing and have given their consent in writing. The blinded evaluation of the study parameters takes place at the University of Ulm, Department of Prosthodontics; the histological evaluation takes place at the University of Bonn, Laboratory for Basic Research in Oral Biology.
2.3 Interventions
Local anesthesia is performed with Ultracain DS 1: 200,000 (Sanofi Aventis, Frankfurt, Germany).
Molars are decapitated and the roots separated with a diamond disk in a dental turbine. A gentle extraction is performed by severing the periodontal fibers with Minvalux instruments (Kohler Medizintechnik, Stockach, Germany) and removing the tooth with a dental forceps after complete mobilization.
The extraction socket is then cleaned by thorough curettage with a sharp curette (Aesculap, Tuttlingen, Germany).
No further socket-related measures are carried out in the control group. After checking that sufficient blood has accumulated in the empty socket, a sterile bite swab is introduced and left in place for 15 minutes.
In the intervention group, a Parasorb Sombrero (Resorba, Nürnberg, Germany) is introduced in accordance with the manufacturer's instructions. The Parasorb Sombrero Membrane Cone is a combination of an absorbable collagen membrane and an absorbable collagen cone in a single product that covers and fills the extraction socket as part of socket preservation. The membrane and cone consist of an equine type 1 collagen without chemical additives or cross-linking agents. A circular supraperiosteal 20 incision is made to prepare a pocket in the coronal soft tissue, using a #15c blade (Aesculap, Tuttlingen, Germany).
2.4 Antioxidant characterization of each meal
The collagen plug, trimmed to the size of the socket, and the trimmed membrane are 1 introduced into the socket without pressure. To prevent the plug from being ejected 2 from the socket, a cruciate mattress suture with Resolon 4-0 (Resorba, Nürnberg, 3 Germany), a monofilament polyamide-6 thread, is placed.
After the extraction, all patients receive instructions for the next 24 hours. Specifically, patients are forbidden:
- To eat while the effect of the anesthesia is still noticeable 7
- To consume alcohol, coffee or caffeinated beverages, and cigarettes or other 8 tobacco products 9
- To rinse the extraction wound, in order to preserve blood coagulate 10
- To manipulate the wound manually (pulling on the lip, massive cleaning of the 11 wound, etc.)
Patients are prescribed 600 mg of ibuprofen for pain reduction, to be taken as individually needed. No prophylactic antibiotic is prescribed.
After 7 ± 1 days, the wounds are visually inspected in all patients. In patients of the intervention group, the sutures are removed at the same time.
A temporary restoration is provided only where essential (for esthetic reasons in the anterior region or for functional reasons in the case of multiple tooth loss), and then only at the request of the patient and while strictly making sure that no direct pressure is applied to the healing socket.
The temporary restorations, where used, are fixed composite provisionals (ProTemp4; 21 3M Espe, Seefeld, Germany), but only if the adjacent teeth are to receive crowns.
If the adjacent teeth are not to be prepared, a removable interim prosthesis made of a thermoplastic resin (Sunflex; Sunflex International, Clearwater, FL, USA) is inserted.
After 6 ± 1 weeks, a precision impression is taken using a polyether impression material 3 (Permadyne Garant; 3M Espe, Seefeld, Germany) to assist in planning the implant 4 position and to document the condition of the alveolar ridge. The impression was taken 5 with a custom tray.
8 ± 1 weeks after the extraction, a CBCT image is taken to support treatment planning. All CBCT images are taken with the same instrument (Gendex CB500; Gendex Dental Systems, Des Plaines, IL, USA) and using the same technical parameters.
After 11 ± 1 weeks, implants are placed (Conelog; Camlog Biotechnologies, Basel, Switzerland). The positioning of the implants is driven by restorative criteria. The virtual planning is carried out using an implant planning software (SMOP; Swissmeda, Zürich, Switzerland). The treatment plan is implemented in a template-driven approach using a 3D-printed drilling stent.
3. Endpoints
3.1 Primary endpoints
- Need for augmentation during implantation. The implants are placed 11 ± 1 weeks after tooth removal. The scope and nature of the procedure and the time needed are documented. In addition to clinical documentation, the need for augmentation is determined from the relation of the original tooth axis and the planned implant axis as a function of the ARP.
- Efficacy of ARP in terms of the preservation of bone. Changes in the alveolar bone are examined by superimposing the digital model data directly after the tooth extraction and the CBCT image 8 ± 1 weeks after the extraction. The metrics of the changes in the horizontal and vertical dimensions are measured at predetermined measurement points.
- Efficacy of ARP in terms of the preservation of the alveolar ridge. Changes in the dimension of the ridge are examined by superimposing the digital model data directly after the tooth extraction and the CBCT image and 6 ± 1 weeks after the extraction.
- Relative cost efficacy of ARP and the standard therapy with unassisted socket healing. The cost of ARP and the necessary augmentation procedures of the post-extraction treatment steps until the time of reentry are determined. The cost can be divided into the cost of material and the cost of the treatment itself. The treatment cost is determined by evaluating (1) the different total treatment time at the times of extraction, implantation and if necessary the augmentation needed and (2) the cost to the patient according to the applicable national dental fee 16 schedule (standard rate).
3.2 Primary endpoints
- Determination of implant survival rates. Control examinations are planned over a monitoring period of five years after prosthetic restoration of the implants.
- Pain following ARP. What is measured is the subjective perception of pain according to the visual analog scale (VAS) one week after tooth extraction.
- Esthetic result of the implant-supported restoration. An evaluation of the pink esthetic score (PES) is performed over the monitoring period of five years.
- Differences between tooth regions (anterior, premolar, molar).
- Patient satisfaction. Monitored by a patient survey using a suitable VAS over the monitoring period of five years.
- Stability of the implant. Measurements of primary implant stability post-insertion (by ISQ) and before delivery of the prosthetic restoration. After delivery of the restoration, stability measurements are made using the Periotest process over the monitoring period of five years.
- Technical complications. Documentation of damage to the restoration, for 8 example chipping or damage at the junction to the implant (screw fracture or 9 loosening) over the monitoring period of five years.
- Clinical findings. Determination of pocket depths (PD) and bleeding on probing 11 (BOP) over the monitoring period of five years.
- Semi-quantitative histological evaluation of the samples taken at the time of implant placement. This evaluation is carried out for a consecutively formed subgroup of the first 10 test and 10 control patients. A subgroup was formed because the sampling process modifies the manufacturer’s recommended drilling 16 protocol and may therefore have an impact on other endpoints when looking at 17 the implants.
3.3 Sample sizes
From a biostatistics point of view, the present study is a pilot study and provides no data that would allow an a priori sample-size determination. It is purely explorative in nature. The sample size was chosen to be similar to comparable studies and is greater than the requirements for inclusion in the previously published meta-analyses on ARP. Following the study, a post-hoc calculation for determining the statistical power of the results will be presented.
3.4 Randomization
Randomization is performed using a computer-generated randomization list (Institute of Epidemiology and Medical Biometry, University of Ulm, Germany). Assignment to the groups is performed by stratified randomization. This randomization 6 is done in 6 layers:
- Gender (two groups: male/female) 8
- Region of the test tooth (three groups: anterior, premolar, molar)
3.5 Blinding
Blinding of the dentist and patient is not possible due to the nature of the therapy itself. Data are acquired, wherever possible, through independent measurement methods (model production after impression, CBCT or photographic documentation). Clinical data collection cannot be blinded because of the study design.
Evaluations are performed in single-blind form. Blinding is ensured through rigorous personal and partial spatial separation from the study center.
3.6 Statistical method
All data are descriptively analyzed statistically in terms of absolute and relative frequencies, median and quartiles or means, and standard deviation and range. Additionally, graphic representations will be used.
Analysis of primary endpoints: The primary endpoints are continuous and will be analyzed by the two-sample t-test or the Wilcoxon test as appropriate.
Analysis of secondary endpoints: The analysis of continuous secondary endpoints will be performed the two-sample t-test or the Wilcoxon test as appropriate. Qualitative secondary endpoints will be analyzed using the chi square test or Fisher’s exact test as appropriate.
Given the exploratory nature of this study, the results from all statistical tests will not be interpreted as confirmatory but in an exploratory way. A result will be regarded as significant if its p value is less than 0.05. No adjustment for multiple testing will be made.
Furthermore, a statistical power analysis and sample-size planning for future studies of this kind will be provided.
3.7 Non-invasive analysis of bone preservation after ARP
The objective of the present method for measuring bone changes after tooth extraction was to develop a procedure that can be performed without having to take radiographs solely for purposes of this study. The clinical therapeutic sequence already provides CBCT images for three-dimensional planning of the implant position and for implementing the treatment plan by means of an appropriate template. Additional images should not be taken for the exclusive purpose of showing the bone configuration immediately after tooth extraction.
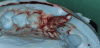
Thus, this non-invasive study protocol calls for an impression to be taken with a hydrophilic impression material (irreversible hydrocolloid) immediately after tooth removal and curettage of the socket (Figure 2). The socket is filled with the irreversible hydrocolloid material using a single-use syringe whose tip has been cut off; the impression itself is a closed-tray impression using the same impression material. Once the irreversible hydrocolloid material has set, the impression tray is removed from the patient’s mouth and disinfected as per the manufacturer’s specifications prior to further processing.
The impression is then scanned in the dental laboratory (3Shape scanner D 700; 3Shape, Copenhagen, Denmark), and a surface record is generated with the inside of the empty socket and the dental arch as reference points (Figure 3).
Eight weeks later, a CBCT is taken to determine the position of the future implant or implants. This pre-implant CBCT documents the condition of bone regeneration or bone resorption eight weeks postextraction.
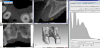
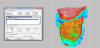
A surface is generated for the DICOM data set that represents the interface of the bone eight weeks post-extraction. The surface is defined in a semi-automated process using a visualization software for voxel data (VGStudio MAX 2.2.5; Volume Graphics, Heidelberg, Germany). In the software, reference materials are specified to delimit the soft and hard tissues. The first step defines the mucosa (Fiure 4), while the second references the target material, i.e., the bone near the extraction site (Figure 5).
The proposed contour of the bone as derived from the calculated grayscale values (Figure 6) must now be controlled and verified. This is done with the help of predefined, clearly recognizable anatomical regions, such as the incisive canal or the hard palate.
Should this result in non-plausible bone surfaces, such as bone being detected outside the bony limits of the alveolar ridge or within the maxillary sinus, the grayscale values are adjusted manually. After determining the contours of the bone, a surface is derived from the data (Figure 7) and stored as an STL file for further processing.
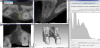
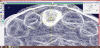
To represent the changes in the bone after tooth extraction, the next step is to overlay the extracted surface from the CBCT with the record of the impression taken at the time 9 of extraction (Figure 8). The reference surfaces for this overlay are the teeth adjacent to the socket to be examined. The teeth are well represented in both data sets and can be regarded as nearly unchanged entities over the period of eight weeks. The superimposition is performed in a software program that is suitable for transferring 3D scan data into accurate polygon models (Geomagic Studio, Version 9; Geomagic, Cary, NC, USA).
The Surfacer software (version 10.6; SDRC Imageware, Neu- Isenburg, Germany) provides a qualitative and quantitative analysis of the agreement or deviation of the point clouds. To this end, the original model from the impression is trimmed to the point 18 where only the bony aspects are shown (Figure 9).
The superimposition then represents the bone apposition/ resorption visually and in 20 quantitative terms (Figure 10). These data sets also allow the definition of fixed measuring distances for linear measurements of any changes in the bone, providing comparability with other methods. The same method can be used to determine volumes.
The linear measurements are obtained after establishing a sextant (Figure 11). In order to use clearly defined measurement points, an asterisk was constructed (yellow color, Figure 11) in the CAD software (Surfacer) and positioned in the impression scan with its center point aligned to the tooth axis as well as one of the three axes orientated in a buccooral direction, dividing the alveoli into sextants. The intersections of the sextant slices with the curves describing the alveolar crest represent the measurement points. These well-defined measuring points cover the clinically relevant regions, providing a connection to values obtained alio loco using analog methods.
3.8 Preliminary results of the feasibility test
Eleven patients were evaluated in this study for feasibility testing of the non-invasive analytical method to determine how ARP preserves the alveolar bone (Table 1). There were cases in the control group and cases in the intervention group available to evaluation. The statistical analysis was carried out using a dedicated statistical software program (SPSS 21; IBM, Amonk, NY, USA). The calculation was performed from the 14 median values and the CI 90%.
There was a median buccal bone loss of 6.2 mm (CI 90%: 0,2–15.9) in the control group and 4.4 mm (CI 90%: 1.3–9.9) in the intervention group. The mean palatal bone loss was 2.5 mm (CI 90%: 1.6–4.4) in the control group and 2.8 mm (CI 90%: 1.4–3.2 in the 18 intervention group. None of the calculated results differed significantly.
4. Discussion
Obtaining CBCT images exclusively for study purposes, without any diagnostic or therapeutic benefit to for the patient, is questionable for ethical reasons. While the probability of radiation-induced tumors following CBCT imaging is rated as low, it is not irrelevant [33]. The method presented here requires no study-related additional CBCT imaging. The only extra step necessitated by the study itself is the reversible hydrocolloid impression after the extraction. This impression requires little additional time on the patient’s part and does not constitute a hazard.
All studies that establishing bone levels based on CBCT images share the problem that bone that is not sufficiently mineralized bone is difficult to detect and that the result is only partially reproducible. The semi-automatic procedure used in the present study reduces the risk of gross misinterpretations as to what might or might not be interpreted as representing bone. The bone contours in the CBCT were determined by a semi-automatic process. One of the advantages of the procedure is that the grayscales can be adjusted individually by detecting tissue classes in the CBCT (soft tissue/mucosa, cortical bone, cancellous bone). In addition, a plausibility check was performed when determining the bone contours based on readily discernible structures (e.g., the hard palate and the nasal spine). Within the range of the expected differences in bone volume changes, the validity of this procedure is somewhat limited due to inherent restrictions.
Leung and coworkers (2010) showed that the marginal bone edge was detectable by CBCT with an accuracy of 0.6 mm and that the measurements were highly reliable [34]. These high intra-rater and inter-rater reliability was also found in another study [35], although that study had examined cadaver heads and did not need to consider the influence of the surrounding tissue, which is non-negligible with CBCT [36], or motion artefacts [37]. Bone structures delimited by cortical bone, however, can be reliably 22 recognized and evaluated [38].
In the present study examining bone changes over 8 ± 1 weeks, the newly formed bone does not completely mineralize in the former socket. No sharp cortical junction of bone and soft tissue is visible in the CBCT [39]. An ambiguous bone boundary is also a clinical problem and cannot be defined in a reproducible way. On the other hand, the microstructure of the trabecular bone can be detected in a CBCT, similar to measurements by micro-CT [40]. This is a fundamental problem in the evaluation of 6 CBCT images, because Hounsfield units (HU) can be correlated with the grayscale values only to a limited extent [41]. An overlay of CBCT data with histological findings could result in higher reliability. Also, CBCT images can be influenced by artifacts mostly metal artifacts and by the imaging parameters chosen. However, the choice of voxel size has only a minor influence on the linear measurement of defined distances [42]. The precision of reversible hydrocolloid impressions after extractions is considered sufficient. The margin of error, which has been given as ± 130 μm [43], is higher than the precision of the CBCT [42].
What is striking in the data is the high level of bone resorption compared to studies that obtained clinical values using a periodontal probe or a caliper [44-47]. In a meta-analysis, values for midbuccal bone height change of –1.0 to –3.5 mm were found in the control groups and –1.1 to 3.5 mm in the intervention groups [10]. These values are significantly different from those found in the present pre-study (–7.0 mm and –5.5 mm in the control and intervention groups, respectively). These differences might be explained by the measurement methodology. Chappuis and coworkers (2013), using their own measuring method in which a CBCT image obtained immediately after extraction was superimposed over a CBCT taken for implant-planning purposes eight weeks later, also found values that were significantly higher than previous results. They found an average midbuccal height change of 5.2 (0.7–12.2) mm and a value that was higher by 3.5 mm than that of the studies to which they referred [14].
The method presented for the measurement of bone resorption after tooth extraction is highly reliable clinically and highly reproducible. Benefits provided by this computer-based method include an exact representation of the initial situation and the radiographic control examination after eight weeks. The method is also characterized by the fact that no additional, strictly study-related radiological examinations are needed. The preliminary evaluation of the patients of the feasibility testing group showed no significant differences between the control and intervention groups. In the literature, however, a positive effect of ARP measures was found in a small number of studies, even though the data available so far have not identified any surgical procedure or biomaterial as clearly superior [48], Other studies, however, showed no evidence of a successful use of ARP procedures [49] There is, consequently, a need for further research, which may be easier to conduct with noninvasive methods such as the method discussed here.
4.1 Status of the Trials
Patient recruitment began on February 25, 2014 and is still ongoing. To date, 69 patients have been included in the study, of whom 37 have had an implant inserted. The recruiting phase will be concluded in December 2016. Follow-ups of the implants are planned for a period of five years. The analyses of primary and secondary endpoints, augmentation needs, bone loss, and dimensional changes of the alveolar ridge as well as the analysis of the health-economic implications is performed promptly after each step of the treatment is performed.
5. Ethics Approval and Consent to Participate
The study protocols were assessed by the local ethical committee. The Ethics Commission of the Medical Faculty of the University of Ulm approved the study design (Docket Nos. 337/12 and 41/14).
All patients gave their written informed consent to participate.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
SiS made contributions to the acquisition of funding, conception, and design, as well as substantial contributions to the coordination of the study, clinical performance, acquisition of data, and drafting the manuscript. ID made contributions to the conception and design of the study, and acquisition of the study. JD made substantial contributions to the conception and statistical design of the study and was involved in drafting the manuscript. HR made substantial contributions to the conception, design, and coordination of the study and was involved in drafting the manuscript. RL made substantial contributions to the acquisition of funding, conception, design, and coordination of the study and was involved in drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Professor Werner Götz, Department of Orthodontics, Oral Biology Laboratory, University of Bonn Germany for the histological examination.
Abbreviations
ARP: Alveolar Ridge Preservation
CBCT: Cone-beam Computed
Tomography
CONSORT: Consolidated
Standards of Reporting Trials
CT: Computed Tomography
PD: Pocket Depth
BOP: Bleeding on Probing