1. Introduction
The administration of antibiotics during bone regeneration process to prevent infection and to accelerate bone healing has been extensively used. However, the localized delivery of antibiotics is severely limited by the high and sustained concentrations of antibiotic that is required in the injured bone tissue. To address these shortcomings, the administration of antibiotics, like ciprofloxacin, from implantable and biodegradable platforms may allow their controlled release, thereby, augmenting the efficacy. The local administration of antibiotics may be facilitated from solid scaffolds with high loading capacities and prolonged release of the drug.
Bone regeneration consists in the formation of new bone after a process of remodeling and constitutes a complex physiological phenomenon that can occur during the repair of fractures. The embryonic tissue and bone are the only ones able to reconstitute itself completely after an injury. Bone is a dynamic tissue that undergoes a constant process of rebuilding consisting of a balance between the osteoid matrix formation by osteoblasts and reabsorption of a quantity of bone by osteoclasts [1]. When the bone tissue is injured, for example after a fracture, a combination of complicated mechanisms with molecular, physiological and biomechanical basis is triggered where the main purpose is the formation of new tissue [2]. However, there are complex clinical situations that can exceed the ability of bone regeneration. We refer to the large bone defects caused by complex fractures, infections, tumors or skeletal deformities. Other diseases involving the bone regenerative process may be the avascular necrosis, atrophic pseudarthrosis, or the severe osteoporosis. Actually, these situations may be managed using bone grafting and bone tissue engineering. These treatments consist of the replacement of the missing bone by autologous or allograft bone or by biocompatible materials. In spite of the success of the strategy, the main drawback is that bone grafting requires surgical interventions for the implantation of the bone scaffold in the injured area, which involves significant health risk for the patient and furthermore, the pathologic processes associated with the bone substitute remain frequent. The biggest risk is the possibility of adherence of bacteria to the scaffold, which might cause an Osteomyelitis. Bacterial contamination probability in hip and knee replacements is the 2%, and 4%. Additionally, these bacterial colonies are very difficult to treat, since microorganisms become more resistant when attached to biological materials [3].
Osteomyelitis is an inflammatory condition of the bone that usually begins as an infection of the medullary cavity and quickly extends to the Haversian system and it finally affects the periosteum of the infected areas. Infections are mainly caused by Staphylococcus aureus and Streptococcus epidermidis. In normal conditions, the healthy bone is very resistant to infection. However, the infection can occur by direct inoculation after a trauma or surgery or it can come from an infected adjacent soft tissue [4]. During the infection, the bacterium produces osteolysis, causing the destruction of the bone that is enhanced by stimulation of osteoclasts through soluble factors released by immune cells after contact with the bacteria [4].
For the treatment of Osteomyelitis, antibiotic prophylaxis is not completely effective, since intravenous administration fails to achieve the required concentrations of drug in bone tissue, bearing in mind that in some cases, very high antibiotic dose can be necessary to achieve effective concentrations in the injured bone. Furthermore, the use of high doses of antibiotics enhances the risk of systemic undesirable effects. In the case of the failure of antibiotic treatment, the choice for osteomyelitis is surgical decortication of the bone and elimination of the necrotic tissue.
One alternative is the local delivery of antibiotics during the orthopedic surgery to reduce the bacterial load at the operative site. For this aim, irrigation solutions, bone cement or cement beads loaded with antibiotics has been used during surgery. Additionally, other methods of local release of antibiotic have been proposed, included powders, pellets, collagen sponges, hydroxyapatite blocks or biodegradable implants [5].
The delivery of local antibiotics for the treatment of musculoskeletal infection is preferable if we place a value on the advantages. Basically, local delivery allows obtaining high local levels of antibiotics in the bone area that are inaccessible by systemic antibiotics. Several studies have demonstrated the benefits of giving antibiotics locally in bone regeneration process together with the implantation of bone substitutes and even though, it is promoted their use mostly in those patients with open fractures or with injuries with a high probability of infection. These studies have shown that the local release of antibiotics decrease the prevalence of the infection and the risk for osteomyelitis associated with open fractures. Use of local antibiotic therapy also minimizes the adverse effects associated with systemic antibiotic therapies in high doses [6]. However, the release of the antibiotic in a controlled manner for long periods of time is not an easy task. In fact, the development of new methods that allow providing high concentration of antibiotic in the tissue surrounding the graft is intended for a more effective therapy. In this context, cyclodextrins are good candidates for their ability to form reversible inclusion complexes with many drugs, thus modifying their chemical, physical and biological properties once the guest molecule is hosted in its cavity [6,7]. The CDs have been approved as excipients for numerous drugs administered by different administration routes. In addition, they can be used to improve various aspects of the release and absorption of drugs, highlighting the increase in bioavailability. Recent studies show that some derivatives of β-CDs can produce anabolic effects [8], with what could be used not only as a method to improve the administration of drugs and prevent the infections but also as material to regenerate bone tissue [8,9].
The development of implant-bone ceramic, polymeric or composite in the form of porous solid structures (scaffolds, matrices or scaffolding) or as injectable bone cement is very important from the point of view of bone regeneration programs [2]. Fundamentally, manufacturing of scaffolds have as principle purpose of controlling the architecture of bone regeneration materials, providing the formation of different structural levels that favours the colonization and development of new bone tissue. Both, the external shape and internal structure are key parameters to achieve the regeneration of damaged tissue. The scaffold must adapt to the place where it will be placed to facilitate the reconstruction of the damaged area while maintaining appropriate mechanical and functional properties. On the other hand, a porous microstructure is fundamental to promote tissue regeneration. Higher porosity and interconnectivity greater facilitate of the proliferation and cell migration, in addition to increased transport of nutrients. The size of the pore diameter which adequate for this purpose is estimated to be between 100 and 500 μm. [2,11] for applications in bone.
One of the possible materials used for the manufacture of scaffolds is hydroxyapatite (HA) (Figure1). HA is an osteoconductive material, which can provide the structure necessary for the formation of bone through allowing the proliferation, migration, and phenotypic expression of cells involved in the regeneration process. [8]. The HA can be used jointly with another series of materials for the manufacture of the Scaffolds and it has been used as a possible support for the local release of antibiotics. [3] However, HA has limited properties of transportation and release of drugs since it tends to be adsorbed on the surface through weak surface interactions.
HA can be obtained from a natural origin or by synthesis. Synthetic production of HA can be made by reaction of aqueous mixtures of calcium sources (CaCL2, Ca (NO3)2, CaCO3 or Ca (CH3COO)2) and phosphates ((NH4)2HPO4, NH4H2PO4, KHPO4, N2HPO4, or NaH2PO4). Synthetics HA results in a porous material with the composition containing calcium, phosphorus and hydroxyl ions (Ca10(PO4)6(OH)2). This material is biocompatible and its structure is similar to the bone. It can be sterilized, it is osteoconductive and displays also an excellent osseointegration [12].
Different methods have been proposed to improve load and drug release properties of HA scaffolds. One of them is the functionalization of the HA with structures that increase the interaction with drugs. CDs seem to be good candidates to improve the transport and release capacity of some antibiotics from HA scaffold [3]. The functionalization of the AH can be achieved by the entrapment and immobilization of CD polymer in the pores and holes of the HA materials as well as by mean physical interactions or chemical bonds [6].
Ciprofloxacin (CPX) is an antibiotic belonging to the group of quinolones of the second generation (fluoroquinolones) of broadspectrum, active against gram-positive bacteria, gram-negative, and mycobacteria. Its main mechanism of action is through interference and in the replication of bacterial DNA by inhibiting DNA gyrase and topoisomerase IV bacterial. In addition, it has a good "aerogynose effect", which means to inhibit the growth of bacteria when the concentrations in the infected tissue are no longer very effective. Due to its broad spectrum, CPX is often used in prophylaxis and treatment of numerous infectious diseases of different organs: respiratory, urinary tract, skin, genitals and include bones and joints. Therefore CPX is a good candidate for use in the bone regenerative therapy [3]. An additional advantage of the CPX is because of its molecular structure is able to interact with CD forming stable inclusion complexes [3].
The aim of this study is to prepare HA microparticles functionalized with CD with improved capacity of transport and release of CPX's to be utilized in the local delivery of the antibiotic for osteomyelitis treatment. The HA microparticles are intended to be used as such or after incorporating them into scaffolds.
2. Materials and Methods
2.1 Materials
Hydroxyapatite (HA); 1,2,3,4-Butanetetracarboxylic acid (BTCA, Merck Millipore, Spain); 1,2- Hydroxypropyl -β-Cyclodextrin (HPB; KLEPTOSE® HPB, M.S. 0.65, ROQUETTE, France); Methanol (Panreac, Spain); Sodium hydroxide (Merck Millipore, Spain), ciprofloxacin hydrochloride provided by Fagron (Spain); Phosphate buffered saline pH 7.4 prepared according to Spanish Royal Pharmacopoeia); All the rest of product are of analytical grade.
3. Methods
3.1 Functionalization of the HA particles with HPB
For the functionalization of the preformed particles of HA, we have used a modification of the method proposed by Martell et al. [3,9]. Dissolutions containing 0.3 g of phosphate disodium, 1 g of BTCA and different amounts of HPB (without HPB, 0.18, 0.50 and 1.0 g) in 10 mL of purified water were prepared. A weight of 1g of HA particles were suspended in the prepared solutions and then were dried in an oven (60-70°C, 24 h) until complete water evaporation. After 24 hours, the samples were pulverized in a mortar and the powder was heated at 150° C for 2 h in a vacuum oven. To eliminate the no reacted compounds, the particles were washed with water and collected by filtration. Finally, the powder was dry in the oven until constant weight.
3.2 Loading of the particles with antibiotic
0.250 g of each CD-HA samples was loaded with CPX by immersion in an aqueous solution containing 1 mg/ml of the drug. Particles were stirred at 100 rpm during 24 h at room temperature. After 24 hours, the powder was filtered, washed with water and left to dry in the oven.
3.3 Preparation of ciprofloxacin loaded HA-HPB microparticles by spray- drying
This methodology was used to synthesize, functionalize and load the HA microparticles in one step.
HA was synthesized in accordance with the reaction:
Cl2Ca•2H2O + Na3PO4•12H2O →Ca10(PO4)6(OH)2 + NaCl+ H2O
Briefly, 4.42 g Cl2Ca•2H2 or 7.27 g Na3PO4•12H2O were dissolved each in 200 mL of purified water. Dissolutions were mixed under stirring and the final solution was introduced in an ultrasonic bath for 1h. The system is let stand for 2 hours until the formation of a suspension was observed that resulted in a flocculated sediment. Then we proceeded to remove the supernatant to eliminate the excess formed of NaCl in the reaction, and the sediment was washed with water. Finally, the sediment was suspended in 400 ml of purified water and divided into four portions of 100 mL. Four batches of particles were prepared:
Batch 1: 100 mL containing 1 g of HA + 200 mg CPX.
Batch 2: 100 mL containing 1 g of HA + 0.5 g BTCA + 200 mg CPX
Batch 3: 100 mL containing 1 g of HA + 1.8 g HPB + 200mg CPX
Batch 4: 100 mL containing 1 g of HA + 0.5 g BTCA + 1.8 g HPB +
200mg CPX
Finally, each batch dissolutions were spray dried (Büchi 190 mini spray-drier, Switzerland) with a flow rate of 5 ml/min, inlet air temperature of 130°C and outlet air temperature of 70°C.
3.4 Determination of the drug loaded in HA microparticles
To assay the CPX loaded in HA particles, two different media solutions have been used: methanol (MeOH) and an aqueous solution of NaOH 0.05N. Methanol solution enables to determine the CPX adsorbed non-specifically in the particles surface, and NaOH 0.5N the complexed drug in the cyclodextrin polymer. For this determination, HA microparticles were placed in 20ml of methanol 24 h at 37°C under stirring. Afterwards, the microparticles are recovered by filtration, dried and suspended in 20 ml of NaOH 0.05N and stirred for 4 h at 37°C. The amount of antibiotic released was measured in a spectrophotometer (Hewlett Packard 8452A) at λ = 280nm (Methanol) or 271 nm (NaOH 0.05N). All the experiments are made in triplicate.
3.5 Drug release study
Samples of 0.050±0.005 mg of HA particles were placed in vials containing 10ml of buffer phosphate saline PH 7.4. The vials were shaken (100 rpm) and incubated at 37°C. Samples of 1ml of supernatant were withdrawn and replaced with 1ml of fresh buffer. Antibiotic concentration was determined by UV spectrophotometry at 271 nm. All experiments were performed in triplicate.
4. Results and Discussion
The HA functionalization method used to immobilize cyclodextrins is based on the methodology developed by Martel et al. [2] for binding cyclodextrins to different textiles and ceramics. It is based on the formation of insoluble polymers of cyclodextrins by esterification with polycarboxylic acid.
In our specific case, we have impregnated the HA particles with a solution of a polycarboxylic acid (BTCA), a soluble cyclodextrin derivative (HPB), and a catalyst (disodium phosphate), to induce the polymerization between the poly-acid and the cyclodextrin at a temperature of 150°C. This resulted in the formation of an insoluble polymer film that it is immobilized on the surface and pores of the HA particles.
Using this technique, different HA particles have been prepared and subsequently functionalized with HPB. Finally, the loading efficiency for CPX and drug release from the functionalized particles haven been investigated as described below.
In this study, Ciprofloxacin has been used as model drug. This drug is useful to be released in the bone tissue to treat the osteomyelitis but also the formation of an antimicrobial coating in the surface of the HA particles can prevent bacterial adhesion and proliferation and in consequence, to enhance the material biocompatibility.
Figure 1 shows the comparison of the CPX loading profiles of the HA particles and those functionalized with the insoluble polymer BTCA-HPB.
The use of methanol for the extraction of CPX incorporated to the HA particles allows to determine the antibiotic adsorbed by nonspecific interactions to the surface of the HA particles, since this solvent is not capable of extracting the molecules bound to the CD [2]. As observed, the levels of CPX adsorbed in the HA surface are slightly higher in the functionalized particles than in those of HA alone (approximately 2 mg/g for HA and 6 mg/ml for HPB-HA particles). No differences were found independently on the amount of HPB used in functionalization. In all cases the amount of CPX adsorbed in the particles is relatively low, suggesting that the CPX has low affinity for HA.
The use of sodium hydroxide as extraction medium significantly increased the amount of CPX extracted from the particles. The basic medium produced the hydrolysis of the insoluble HPB polymer that led to the release of the complex formed between the CPX and the HPB, increasing the concentration of antibiotic in the medium. As can be seen in figure 1, in the case of unmodified HA, no CPX was detected after sodium hydroxide extraction. This is indicative that all the molecules bound to the HA has been released in the methanolic medium. However, the functionalized HA particles gave rise to levels of bound CPX higher than 50-60 mg per g of particles although no differences were founded between those functionalized using different concentrations of BPH. These results suggest that using the lower concentrations of HPB was sufficient for the formation of the insoluble HPB polymer on the surface of the particles by coating entirely the surface and porous of HA particles and thus, allowing for the CPX load to be increased more than 40 times with respect to the non-functionalized HA particles. This result confirms that The CPX interacted strongly with HPB polymers facilitating its loading. It has been described previously that ciprofloxacin is able to form inclusion complexes with stable βCD [12].
The release of CPX from the HA particles and the three HA-HPB functionalized particles in phosphate buffer is shown in Figure 2.
A controlled drug release was observed from all the functionalized HA particles with the total amount of the drug released in the period of 48 hours. Furthermore, in all CPX-CD-HA particles an initial relatively faster release profile was observed during the first 10 hours. After, a slight decrease from this time was prolonged in a slow manner until 48-72h, since from that point the rest of the loaded drug was completely released. As in the loading experiments, no differences were detected with the percentage of HPB used. Nevertheless, for unfunctionalized HA-particles the complete release took place much more rapidly, just in a few minutes, and in lower proportion, thus indicating that the particles were unable to control the release of CPX. In accordance with the results, the particle functionalization using 1.8% of HPB was selected for next experiments.
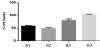
In a second stage of the study, HA particles with the cyclodextrin polymers and loaded with the antibiotic were fabricated in one-step using spray drying methodology. To prepare particles, four groups of particles were been synthesized: two types of microparticles were obtained, first of them without functionalizing (B1 and B3) and a second formulations displayed functionalization (B2 and B4) with (B1 and B2) and without HPB (B3 and B4).
Microscopic observation of the hydroxyapatite microparticles (figure 3 and 4) revealed different morphologies depending on each composition. The observation of both samples directly (scanning electron microscopy, figure 4) and the samples in aqueous dispersion (optical microscopy, figure 3) revealed the existence of spherical particles, although irregular surface was observed only in the formulations B1 and B4. In these samples also the presence of CPX crystals of acicular shape was easily visualized, isolated and adhered to the surface of the particles. The HA microparticles obtained showed a size between 1-5 μm and a very irregular surface, with pronounced invaginations. On the other hand, formulations named as B2 and B3 were aggregated in irregular particles of different size, so the visualization of isolated particles was negligible.
Figure 5 shows the loading drug profiles of each type of particle. As can be observed after extraction with sodium hydroxide, the particles containing HPB displayed the best capacity of immobilization of the CPX and showing slightly higher amounts of loaded drug when the HPB and BTCA was incorporated. As observed, the formation of the inclusion complex between the HPB and CPX may favor the immobilization of the drug in the HA-microparticles. In addition, the formation of the insoluble polymer of cyclodextrins during the formation of the particles could facilitate the retention of the drug inside the polymer.
Figure 6 shows the release profiles of CPX. As can be seen, the release of CPX from the different types of particles was extremely rapid (practically all the drug has already been released after 10 minutes), being a little more gradual in the B4 particles. The particles prepared in the absence of cyclodextrin, B1 and B2, displayed a much slower proportion of drug released than B3 or B4. CPX has very low water solubility at pH 7.4, so the formation of inclusion complexes with cyclodextrins, which has a markedly superior solubility [12], significantly improved the release. The differences between B3 and B4 may be due to the higher proportion of cyclodextrin incorporated by B3 which may increase the solubility of CPX in the dissolution medium. Similarly, the increase in B4 release suggests that these particles also delivered cyclodextrin into the medium, so in this case, either the hydrolysis of the insoluble polymer occurred or under the conditions used during atomization, the complete functionalization of the particles was not achieved remaining therefore, a large part of the cyclodextrin as a free product. From the data obtained, we believed that HA-CD functionalized polymer might represent an interesting technological platform for the local delivery of antibiotics in the context of bone regeneration therapy. Nevertheless, a major research into this approach is required to complete the development of novel strategies based on the formation of the insoluble polymer and to obtain a more prolonged release rate of poor soluble antibiotic drugs.
5. Conclusion
In this work, an approach based on hydroxyapatite functionalized with cyclodextrins for the loading and release of an antibiotic drug (ciprofloxacin) has been developed with bone regeneration purposes. A polymer composed on 1,2-Hydroxypropyl-β-cyclodextrin and hydroxyapatite was successfully synthesized although the cyclodextrin concentration used for the preparation did not render in more loading of cyclodextrin. Particles obtained by spray-drying showed different morphologies depending on the composition and the inclusion complex with ciprofloxacin could be improved by the addition of BTCA during the drug loading. The use of cyclodextrin in combination with HA clearly increased the drug loading and slightly improved the release pattern of ciprofloxacin compared to the HA alone. Due to the high loading obtained, this novel polymer seems to be a good platform for the prolonged release of drug during bone regeneration therapies, although the formulations of HA-CD on their own are probably insufficient to maintain the long periods of release for the usual treatment of several weeks that is required in bone healing.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
B.R.A., A.G.G. and A.F.D. design, acquisition of data and analisys
of data.
J. B.M., A.L.A. and F.O.E. conception, analisys and interpretation
of data.