1. Introduction
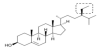
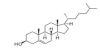
β-Sitosterol (beta-sitosterol; Figure1) is a natural phytosterol. It is a steroidal molecule similar to cholesterol (Figure 2) but is of a plant origin. Because of its steroidal lipophilic nature, it is present in many oils from plants and vegetables. It attracted attention of scientist because it may affect cholesterol absorption [1]. Consequently, it was thought that it decreases the risk of cardiovascular diseases [2]. This review is devoted to claims of β-sitosterol effects on various types of cancer [3].
2. Method
The review is based on data obtained from PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and from PubChem Compounds (http://www.ncbi.nlm.nih.gov/pccompound/) with preference given to the data obtained during the last 5 years. The search terms were varied. The main search terms were: β-sitosterol, beta-sitosterol, cancer, analysis, determination, in vitro, in vivo, anti-cancer activity, breast cancer, colon cancer, prostate cancer, diagnosis, marker, biomarker, therapy, diet or dietary, carcinogenicity, chemoprevention and various combinations of these terms.
3. Chemical Properties of β-Sitosterol
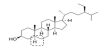
β-Sitosterol (C29H50O, molecular weight 414.71 g/mol) is an optically active steroidal compound. Optical activity is reflected in its chemical name is (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta [a] phenanthren-3-ol. This molecule contains only one functional group – a secondary alcohol and one double bond that may be saturated. This double bond saturation results in the formation of another sterol compound - stigmastanol (Figure 3). The chemical structure of β-sitosterol was investigated and reported as early as in 1931 [4].
Despite low reactivity (only one alcoholic group and one double bond in the structure, various derivatives of β-sitosterol were patented for the use in therapy of obesity, [5] diabetes, [6-8] syndromes associated with diabetes, “such as treatment and control of hyperglycemia, as well as dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, and obesity” [9] and, importantly, with cancer [10].
4. Analytical Determination of β-Sitosterol
In the age of modern instrumental methods, the analysis of relatively complex molecules of various structurally similar phytosterols is usually performed using chromatography separation of analytes combined with some type of spectrometric detection. There are many scientific reports on determination of phytosterols in various plants, either using gas chromatography [11-13], liquid chromatography [14-16], or both methods [17]. However, only few reports are available on determination of phytosterols in biological systems of non-plant origin.
Cellular uptake of β-sitosterol was investigated using Caco-2 cell with means of ultra performance liquid chromatography using ultraviolet detection (UPLC-UV), ODS column and mobile phase consisting of acetonitrile and water [18]. The uptake of β-sitosterol and other phytosterols was shown to be time- and concentrationdependent.
As cholesterol presence in biological fluids serves as an indicator in various morbid situations, attention is being paid to the sample processing time. The determination of sterols (free and bonded), including β-sitosterol, in human serum is probably the most important in this regard.19 Total sterols are determined using the assistance of ultrasound to decrease the time of the alkaline hydrolysis followed by solid-phase extraction. Coupling of liquid chromatography to mass spectrometry represents a modern, instrumental approach to analytical determination of free and bonded sterol precursors, phytosterols that are similar in their chemical structure.19 In another work, β-sitosterol concentrations in plasma were measured as an indicator of cholesterol absorption in healthy volunteers using LC-MS method [20,21]. It was shown in 263 patients that there is no association between β-sitosterol concentrations the lever fat content (LFC) [21]. The metabolic turnover of β-sitosterol and other pharmacokinetic parameters were studied in healthy subjects using [(14)C]β-sitosterol [22]. Steady-state concentrations of β-sitosterol was determined to be 2.83 μg/ml and the calculated dietary load of β-sitosterol was determined to be approximately 1400 mg/day [22]. It is now fully established that β-sitosterol and other sterols are in a relation to the metabolism of cholesterol [23]. GC-MS (gas chromatography-mass spectrometry) method was used to show this in hyperlipidemic patients as studying cholesterol metabolism before and after therapy (for example with statins) and using sterols turnover in hyperlipidemic patients might be useful in identifying the best for a specific patienthypolipidemic treatment [23]. Asimilar sensitive and specific isotope dilution GC-MS method was applied in the analysis of oxygenated β-sitosterol (and campesterol) that may eliminate the primary anti-atherosclerotic action of cholesterol lowering [24]. The results of this studyhave shown the suitability of this instrumental method for detection of phytosterols and their oxygenated products at extremely low concentrations [24].
A new, progressive method based on GC-MScoupled with extraction using β-sitosterol magnetic molecularly imprinted polymer (mag-MIP) beads was developed and applied [25]. The method is suitable for analysis of complex biological matrices. β-Sitosterol mag- MIP beads were prepared by a rapid microwave synthesis. β-Sitosterol mag-MIP beads were shown to possess a high enrichment factor for β-sitosterol (approximately 20-fold) and improved β-sitosterol selectivity [25].
An additional modification of GC-MS method is represented by mass spectrometry-selected ion monitoring [26]. This was used in determining concentrations phytosterols and their oxidized products in plasma and tissue. Such analyses have a strong potential in determining the cardio-vascular risk of an individual patient [26].
5. Pharmacokinetic parameter of β-sitosterol
Only few reports deal with pharmacokinetics of β-sitosterol. Recently, pharmacokinetic of β-sitosterol was studied in neonates and in normal newborns [27]. In this study, β-sitosterol and other phytosterols (stigmasterol and campesterol) were from soybean oil. Soybean oil infusion resulted in β-sitosterol steady-state plasma concentration of 1.68 mg/dl to be reached within 36 hours. It was determined that accumulation of β-sitosterol and other phytosterols is increase in neonates, probably due to immature mechanism of elimination [27]. Phytosterols having minimal potential for adverse effects in the organism after oral consumption because of their low bioavailability but may have some effects, if absorbed. Plasma concentrations of β-sitosterol after oral administration were established with the β-sitosterol dose of 10.7 mg per experimental rat (250 g; the dose was based on an anticipated human total phytosterol consumption of 3 g/day and an estimated body weight of 70 kg) divided to 4 parts and administered orally at 8, 16, 24, and 32 hours from the beginning of experiment. The determined concentrations of β-sitosterol were 8.87 ± 0.04 μg/ml after 8 hours, 12.63 ± 1.00μg/ml after 16 hours, 12.67 ± 0.91μg/ml after 24 hours and 9.92 μg/ml after 32 hours (no standard deviation is shown as only 1 animal reached this time point). AUC0–32h (area under curve from the time 0 to 32 hours) determined for β-sitosterol was 293.20 ± 43.7μg/ml.h [28].
It was demonstrated recently that β-sitosterol may enter the brain [29]. Additionally, the reversibility of β-sitosterol accumulation in various organs was investigated. The obtained data shown that concentrations of β-sitosterol (and campesterol) increased 2-3-times in serum, liver and brain when the experimental mice were on a phytosterol-rich diet. Elimination of these plant sterols from diet resulted in normalization of β-sitosterol (and campesterol) concentrations in serum and liver. However, concentrations of phytosterols in the brain were not decreased even after 6 months and it was concluded that sterol accumulation in the brain is de facto irreversible [29].
6. β-Sitosterol in vitro
While β-sitosterol newer achieved the stage of clinical investigation. This is despite many reports of its anticancer activity in vitro in various cellular systems, such as HT-29 cells, where β-sitosterol demonstrated 2.6 times higher selectivity than methotrexate [38]. β-Sitosterol is clearly recognized as a valuable component of human diet that possesses anticancer or cancer preventive properties/effects due to its interaction with various cellular targets and pathways. However, this wide recognition of cancer chemopreventive properties occasionally lacks in strong scientific evidence. It is possible that the ability of β-sitosterol to affect the cholesterol-related processes in the body may overlap with its anti-cancer actions [39]. β-Sitosterol may inhibit P-glycoprotein,a membrane transporter encoded by the MDR1 gene in human cells mediating drug efflux. P-glycoprotein plays a key role in the phenomenon of multidrug resistance (MDR) due to the cellular efflux of anticancer therapeutics from human cancer cells. MDR is in many cases responsible of an unsuccessful chemotherapy as a result of MDR in cancer [40]. MDR reversal in cancer cells may be achieved by combining digitonin and β-sitosterol (orsome other secondary metabolite) through a significant down-regulation of MDR1 as shown in in vitro experiments [40].
It was shown in ovariectomized athymic mice (and also in vitro) that β-sitosterol, β-sitosterol glucoside or a mixture of these two compounds modulate the growth of estrogen-responsive breast cancer cells. Mice had these compounds in their diet (β-sitosterol 9.8 g/kg of mice weight; β-sitosterol glucoside 0.2 g/kg of mice weight) for 11 weeks. No stimulation of MCF-7 tumor growth was detected. On the other hand, the significant reduction of MCF-7 tumors by β-sitosterol(38.9%) and byβ-sitosterol glucoside (31.6%)were recorded. Additionally, expression of the antiapoptotic marker bcl-2 in tumors was downregulated [41].
Breast cancer patients may benefit from findings that dietary β-sitosterol enhances tamoxifen effectiveness [42]. Additionally, the study of the effect of dietary phytosterols on the growth and formation of metastasis of the human breast cancer cell line MDA-MB-231 as a xenograft in SCID mice shown 8 weeks after tumor cells inoculation showed that animals fed with phytosterols had 20% less lymph nodes and lung metastases and the size of tumors was decreased by one third. These results led to a conclusion that dietary phytosterols (including β-sitosterol) inhibit the growth and metastazing of breast cancer cells [43].
SCID mice were also used in the study of dietary effects of phytosterols (compared to cholesterol) on the growth and metastasis formation of the PC-3 human prostate cancer cell line [44]. The experiment was similar to the one described earlier [43]. Tumors in animals on phytosterols diet were 40% smaller compared to cholesterol-fed control. Additionally, number of animals with lymph node and lung metastasis was decreased by one half of the control cholesterol group. The protective/inhibitory effects of β-sitosterol were significantly higher compared to other phytosterol – campesterol [44].
In the study of colon cancer, β-sitosterol was shown to serve as an effective substance reducing oxidative stress in vivo and thus serving as a chemopreventive agent in colon carcinogenesis [44]. The experiments were based on induction of colon carcinogenesis by 1,2-dimethylhydrazine (DMH). DMH increased liver lipid peroxidation. β-Sitosterol was capable of effectively protect the organism of experimental animals against DMH-induced depletion of catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, glutathione S-transferase, and reduced glutathione in colon and liver of experimental rats [45].
7. β-Sitosterol as a biomarker for colon cancer chemoprevention in human
β-Sitosterol was relatively extensively investigated for its possible use as a therapeutic agent in cancer. This investigation, despite bringing many important pieces of information regarding its biological role and potential benefits, did not result in introduction of this substance among anti-cancer therapeutics. However, beneficial data were obtained indicating the possible role of β-sitosterol as a biomarker.
Recently, an interesting study performed in mice shown that daily administration of β-sitosterol activates immune response of the organism. This was reflected in activity of cytokines IL-12 and IL- 18 and natural killer (NK) cells and led to significantly decreased number of metastases in in vivo experiments with lung cancer cell line. Mechanisms of this effect is possibly based on enhancement of gut immune surveillance systems [46]. Low degree of β-sitosterol absorption slows colonic epithelial cell proliferation that results in decreased expression of neoplastic transformations [47]. These results are no surprising as it was already shown that incidence of some cancers, i.e. of breast cancer, vary among various human populations in the relation to their level of phytosterol consumption.48 It was shown in more than hundred volunteers that β-sitosterol can serve as a biomarker of exposure in observational or compliance studies of cancer prevention [48], especially because the high reproducibility and reliability of β-sitosterol determination in plasma [49]. Carcinogenic and cancer-related processes are reflected in production of fecal neutral steroids in colon [50]. The concentrations of fecal neutral steroids in colon were increase in colon cancer cases when compared to healthy controls (57.3 ± 2.4 vs. 36.9 ± 3.5mmol/kg of wet feces). The data obtained indicate the relationship to malignant potential of colon cells and may be valuable in assessing cancer risk of the colon cell donor [50].
8. Conclusion
Phytosterols, including β-sitosterol, are recognized for their role in human nutrition and for their anticancer potential. Development of modern instrumental analytical methods brought new options in investigating fate and mechanisms of activity of β-sitosterol in human organisms. It is now clear that β-sitosterol affects many metabolic systems in the body. However, despite its clearly beneficial role as an anticancer agent, two main factors are in the way of the use of β-sitosterol in cancer therapy: 1) Its relatively low activity; and 2) similarity to cholesterol with its presence in daily diet. The ability of β-sitosterol to affect the cholesterol-related processes possibly overlaps with its anti-cancer actions. Additional elucidations of the role of β-sitosterol in human organism may contribute towards its recognition as a beneficial substance or agentthat benefits some specific patients’ groups, such as breast or colon cancer patients at least in the form of a diet supplement.
Competing Interests
The authors declare that they have no competing interests.