1. Introduction
Losartan 50 mg resulted in significant reductions of systolic and diastolic blood pressure at 24 hours after dosing. Approximately 70% of the maximal reduction had occurred by one week of therapy [1]. In patients with type 2 diabetes mellitus, Angiotensin II receptor blockers (ARBs) are especially effective for decreasing nephropathy progression rate, independent of their blood pressure–lowering effect. In the Reduction of Endpoints in NIDDM (noninsulin–dependent diabetes mellitus) with the Angiotensin II Antagonist Losartan study (RENAAL study), Losartan reduced the risk of the doubling of serum creatinine concentration by 25% and of end stage renal disease (ESRD) by 28% [2].
The decreased risks of ESRD (26%) and or death (19%) remained unchanged after adjustment for blood pressure, indicating renoprotection was independent of blood pressure reduction [2]. Losartan has been found to down-regulate the expression of transforming growth factor beta (TGF-β) types I and II receptors in the kidney of diabetic rats, which may partially account for its nephro-protective effects [3].
2. Material and Methods
2.1 Animals
Male albino rats weighing (130±20 gm) were obtained from the animal house of Al-Nile Pharmaceutical Company. They were housed in stainless steel cages under a 12 hours light / dark cycle at room temperature. Animals were kept under the same condltion with regard food and water all over the period of this study. Each individual animal was weighed before start of therapy and was clearly marked by gentian violet to indicate its weight. The doses of drugs were accurately calculated according to the weight of each animal.
2.2 Apparatuses
- Centrifuge.VEB MLW Zentrifugenbav engelsdorf type T52.1.
- Spectrophotometer “Shimadzu, UV-visible recording spectrophotometer UV-160, Shimadzu Corporation Kyoto-Japan”.
2.3 Drugs and chemicals
In this study, the following drugs and chemicals were used:
- Gentamicin sulphate 80 mg /ml (Epigent) was obtained from: E.I.P.I.Co.
- Losartan (50 mg. tab. - Amriya comp).
- Urea and creatinine kits.
2.4 Induction of Nephropathy in Rats
The rats received ordinary rat diet for 6 weeks and were given gentamicin injection in a dose of 80 mg/kg intraperitoneal (IP) once daily) [4] during the last 8 consecutive days.
2.5 Study design
Animals were randomized after estimation of basal blood urea and serum creatinine into four groups as follows:
2.5.1 Group I (Control group)
10 rats received ordinary rat diet for 6 weeks, received Na Cl 0.9% intraperitoneal (IP) in equivalent volume as gentamicin treated rats in the last 8 consecutive days, and served as control group.
2.5.2 Group II (Gentamicin group)
10 rats received ordinary rat diet for 6 weeks and were given gentamicin injection in a dose of 80 mg/kg intraperitoneal (IP) once daily [4,5] during the last 8 consecutive days.
2.5.3 Group III (Losartan group)
10 rats received ordinary rat diet for 6 weeks and receive Losartan10 mg/kg/day orally by gastric tube concomitant with the ordinary diet [6].
2.5.4 Group IV (Losartan and gentamicin group)
10 rats received ordinary rat diet concomitant with Losartan 10mg/ kg/day orally by gastric tube for 6 weeks, and gentamicin injections (80 mg/kg/day) intraperitoneal (IP) once daily in the last 8 consecutive days [7].
2.6 Samples collection
The animals were anaesthetized using diethyl ether. Blood samples from the venousplexus deep to the medial canthus of the palpebral fissure. 5 ml blood were added to an anticoagulant ( EDTA) centrifuged at 2,000 rpm for 10 min at 4⁰C to separate plasma from erythrocytes. The biochemical parameters were determined as follows.
2.7 Biochemical analysis and technique
- Serum urea (measured in mg/dl).
- Serum creatinine (measured in mg/dl).
2.8 Determination of serum urea
Blood urea levels were determined following urease modifled berthelot reaction [8] using kit supplied by Biomerieux- France.
2.9 Reagents
- Reagent 1 (standard): urea 50 mg/dl.
- Reagent 2 (enzyme): urease
- Reagent 3 (color reagent): phosphate buffer (pH = 0.8), sodium salicylate, sodium nitroprusside and ethylenediaminotetra-acetic acid (EDTA).
- Reagent 4 (alkaline reagent): sodium carbonate and sodium hypochloride
- Working reagent: one bottel of reagent 3 was reconstituted with one vial of reagent 2 and shacked gently.
2.10 Procedure
- 1 ml of working solution was pipetted into 3 test tubes (test, blank and standard)
- 10 μl of tested serum sample and 10 μl of standard solution were added to their corresponding tubes.
- The tubes were mixed and incubated at 37⁰C for 3 minutes.
- 200 μl of reagent 4 was added to each ofthe three tubes.
- The tubes were mixed and incubated at 37⁰C for 5 minutes
- The absorbance was read using a spectrophotometer at wave length of 580 nm and setting the blank at zero.
2.11 Calculation
2.12 Determination of serum creatinine
Serum creatinine was determined by kinetic measurements according to the methods of [9] using kit supplied by Human-Germany.
2.13 Principle
The complex formed by creatinine and picric acid in an alkaline medium is measured for two minute
2.14 Reagents
- Reagent 1 (standard): creatmine 2 mg/dl.
- Reagent 2 (colour reagent): picric acid.
- Reagent 3 (alkaline reagent): sodium hydroxide diluted with distilled water in the ratio 1:4.
- Working reagent: one volume of reagent 2 was mixed with one volume of reagent 3.
2.15 Procedure
- 1 ml of working solution was pipetted into 2 cuvettes (test and standard).
- 100 μl of tested serum sample and 100 ul of standard solution were added to their corresponding cuvettes.
- The cuvettes were mixed and stopwatch was started immediately.
- The absorbance was read using a spectrophotometer at wave length of 492 nm between t= 20 sec and t= 80 sec where the zero adjustment was the air.
2.16 Caculation
ΔA= A2-A1
A2: absorbance 60 seconds after first measurement A1.
A1: absorbance at 20 seconds.
2.17 Collection of kidney for histopathological examination
At the end of 6th week after drug or vehicles administration the animals were sacrificed and the kidneys were removed for histological and ultra structure examination. One kidney was cut longitudinally; one half was fixed in 10% buffered formalin and embedded in paraffin. Sections of 5-mm thickness were cut and stained with Mayer's hematoxyline and eosin (for examination of cell structure) and examined by light microscope [10].
2.18 Statistical analysis of results
The variability of results was expressed as the mean ± standard deviation (X ± SD). Statistical analysis of the difference between groups was performed by using Statistical package for the social Sciences (SPSS) version 20, using one-way analysis of variance (ANOVA), and paired T test. Charts were done using Excel program, Microsoft Office XP 2007.
2.19 Degree of significance
P > 0.05 = insignificant difference.
P < 0.05 = significant difference.
3. Results
3.1 Serum urea
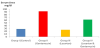
The mean of serum urea levels of the control group was 28.2±1.5mg/ dl, and in gentamicin treated group was 92.3±1.2mg/dl. This level showed a significant increase compared to the control group (P<0.05) (Figure 1).
Our study found that in Losartan treated group the mean serum levels of urea was 25.2±0.9mg/dl. This level showed no significant change compared to control group (28.2±1.5mg/dl), This study found that gentamicin and Losartan treated group, thier mean serum urea levels was 60.4±1.2 mg/dl. The statistical analysis indicate that the serum levels of urea showed a significant decrease as compared to that levels of gentamicin treated group which was 92.3±1.2mg/dl by using paired T test the P value < 0.05.
3.2 Serum creatinine
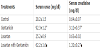
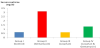
Our results expressed as mean ± SD (Table 1, Figure 2) show that in the control group, the mean of serum levels of creatinine was 0.62±0.05 mg/dl, and in group treated with gentamicin ,the mean of serum levels of creatinine was 2.7±0.09 mg/dl. This level showed a significant increase compared to control group (P<0.05).
The results obtained for group treated with Losartan, the mean of serum levels of creatinine was 0.63±0.03 mg/dl. This value showed no significant change compared to control group. In gentamicin and Losartan treated group, the mean of serum levels of creatinine was 0.98±0.08 mg/dl, this value showed a statistically significant decrease compared to gentamicin treated group (P<0.05). The results proved that Losartan has a nephroprotective effects.
3.3 Histopathology
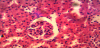
In the control group, section of rat kidney shows normal appearance of glomeruli and tubules, (figure 3). In gentamicin treated group, section of rat kidney shows dilatation of the tubules with drop out of some epithelial lining (picture of tubular necrosis), (figure 4).
In Losartan treated rats, animals show normal appearance of glomeruli and tubules. While, that treated with gentamicin and, Losartan show apparently minimal glomerular atrophy and minimal tubular heamorrhge.
4. Discussion
The present study was designed to investigate the possible potential renoprotective role of Losartan in gentamicin induced nephrotoxicity in rats.
Rats are suitable models of experimental animals for studying the gentamicin nephrotoxicity. This was evidenced when toxic effects of gentamicin on several enzyme activities of kidney were compared between rats and mice, it was found that rat serum urea concentration was significantly increased in gentamicin nephrotoxicity while no change occur in mice [11].
In the present work histopathologically (in gentamicin injected animals), the kidney sections showed dilatation of the tubules with drop out of some epithelial lining, (picture of tubular necrosis), (figure 4).
In accordance with [12] our results in sections from control group showed normal histological structure of the glomeruli and renal tubules in the cortex and normal tubules in the medulla, (figure 3).
But in renal sections from gentamicin-treated rats, the glomeruli showed atrophy in some of them and hypertrophy in others, also there were degeneration and necrosis in the epithelial cells lining the renal tubules with cystic luminal dilatation in others at the cortex. Mononuclear leucocytes inflammatory cells infiltration was observed in focal manner between the tubules in the corticomedullary junction as well as in the perivascular area of the dilated blood vessels associated with edema, (figure 4).
These results were in line with the result obtained by Naidu et. al. and Joel et. al. [4,13] they reported that gentamicin treated rats showed in addition to the necrosis of proximal tubules formation of hyaline casts and dilatation of distal tubules. In addition Sandhya et al. [14] reported that gentamicin treated rats showed the presence of homogenous materials in the form of droplets of masses in proximal convoluted tubules in addition to inflammatory filtrates in the interstitium and these changes were markedly reduced by lipoic acid treatment.
In the present study Losartan administration, to the rats injected with gentamicin showed marked improvement of renal function (significant decrease in serum urea and creatinine). The previous results agree with [15] who showed that treatment with Losartan significantly reduced blood urea nitrogen (BUN) and serum creatinine levels elevated by gentamicin administration. Also, Losartan significantly attenuated gentamicin -induced increase in malondialdehyde and decrease in reduced glutathione, catalase and superoxide dismutase activities in renal cortical homogenates which is proved by Hall A et. Al. [16], and Herrera et. al. [17] they found a strong correlation between loss of peroxiredoxin2 (Prdx2) and podocyte apoptosis and proteinuria was also demonstrated in the Ang II–infused rats.
Additionally, histopathological examination and scoring showed that Losartan markedly ameliorated gentamicin -induced renal tubular damage.
On the basis of previous results, it is proved that Losartan has great renoprotective effects, so it is advised to use Losartan in patients having renal troubles especially in ischemic nephropathy and hypertension.
Acknowledgments
We take this opportunity to show our greatest appreciation to Dr. Zeinab Alkasaby Zalat.