1. Introduction
Legionnaires’ disease (LD), usually is acquired by inhalation or aspiration of aerosol contaminated by Legionella species from environmental water sources, such as hot water supplies, cooling towers, and evaporative condensers. Potable water is considered as an important infection source in community-acquired, nosocomially acquired, and travel-associated LD cases [1-8]. Legionella spp. are gram-negative bacteria that normally occupy natural aquatic environments, where they can survive as intracellular parasites of protozoa. It includes 52 species and over 70 different serogroups. Over 20 species have been proven to be causative agents of LD. However, Legionella pneumophila species accounts for approximately 90% of confirmed cases of legionellosis. The majority of community acquired cases are caused by strains belonging to Legionella pneumophila serogroup 1, other non-L. pneumophila sg 1 strains, sg 2 to 15, accounted for 7.4% of cases [9,10]. The genetic diversity of L. pneumophila was related to horizontal gene transfer of mobile genetic elements among L. pneumophila strains, and between different Legionella species. The potential health risk of Legionella to humans is theoretically associated with cells densities above 104 to 105 CFU per liter of water [11,12].
Commonly used method for environmental surveillance of Legionella is the standard culture technique [13,14]. Legionella culture is required for epidemiological typing of isolated strains to detect the source of the infection [15,16,17]. However, the fastidious nature of these bacteria, effect the culture technique’s sensitivity to be 30 to 60% and require 3 to 7 days to grow visible colonies. Therefore, numerous phenotypic and genotypic typing methodologies have been developed and applied to the epidemiological typing of L. pneumophila [18]. These methods included monoclonal antibody (MAb) subgrouping method [19] and genotyping methods, such as restriction fragment length polymorphism (RFLP) analysis [20], amplified fragment length polymorphism (AFLP) [21], and pulsed field gel electrophoresis (PFGE) [22,23]. Recently, a sequence-based typing (SBT) scheme of L. pneumophila that uses sequences from seven genes has been used in typing L. pneumophila serogroup 1 strains, and strains belonging to others serogroup [16]. The SBT method has the potential for excellent type ability, reproducibility, and epidemiologic concordance [16, 22- 24] and now is established as the standard sub typing technique within the European Working Group for Legionella Infections (EWGLI). The strains are assigned an SBT pattern number by EWGLI (EWGLI: www.ewgli.org) based on the sequence of the seven target genes. The method is now considered the gold standard for epidemiological typing [16,24].
In Kuwait, research related to Legionella is scarce and no active surveillance program exists [25-27]. Furthermore, no reports are available on the current status of the prevalence Legionnaires’ disease and associated cases in Kuwait. However, annual reports presented by the Ministry of Planning show that the death of a percentage of the population (27.6/104 of population) is due to respiratory diseases without specifying the etiological agent [28].
Due to the following factors, it is likely that the prevalence of Legionella is underestimated; Kuwait’s a hot temperate climate; the absence of water safety regulations for Legionella monitoring and decontamination; and the increased use of cooling towers within recreational and health care facilities may increase the risk of legionellosis. In addition, water temperatures in water tanks during the summer in residential compounds may be favorable for Legionella multiplication (50oC). Owing to the possibility of environmental exposure to Legionella, this is the study aimed at assessing the current distribution of Legionella species from environmental water sources from public facilities such as buildings, public baths, hospitals, throughout Kuwait. Furthermore, the molecular typing of L. pneumophila sg isolates was conducted using sequence-based typing (SBT) to assess the genetic diversity among the isolates.
2. Material and Methods
2.1 Legionella pneumophila
A total number of 102 environmental isolates of L. pneumophila were used in this study. These isolates were previously isolated from domestic water system samples from different residential sites [29] and from 38 cooling towers in 12 sites in Kuwait [30]. L. pneumophila was isolated on buffered charcoal yeast extract medium using standard methods AS/NZS 3896 [31].
2.2 Sero and subgrouping of Legionella pneumophila
The Oxoid Legionella Latex test was used to identify and differentiate between sero group 1 and serogroups 2-14 (code DR0800; Oxoid; UK). The isolates were sero grouped and sub grouped, when applicable using the Dresden panel of monoclonal antibodies aspreviously described [9,10].
2.3 Sequence based typing (SBT)
The genomic DNA was extracted from the isolates using the QIAamp DNA Mini kit (Qiagen). L. pneumophila isolates were genotyped using the seven gene protocol sequence-based typing (SBT) scheme developed by ESGLI as previously described [16,24]. Trace files with the obtained sequences were analyzed by using the Legionella SBT quality tool. New alleles and STs encountered for the first time in this study were submitted to the database.
2.4 Whole genome sequencing (WGS)
The genomic DNA was extracted from four representatives isolates using the QIAamp DNA Mini kit (Qiagen) and was subjected to whole genome sequencing using the Illumina MiSeq platform with 250 bp paired-end reads according to the manufacturer's instructions. The flaA genes were extracted from the de novo assemblies (CLCbio vers. 8.0). The four isolates were selected among isolates that failed to give aflaA PCR product and showed one of the following four allelic profiles(1) F,14,16,16,15,13,2; (2) F,14,16,25,7,13,24; (3) F,14,16,65,7,13,217; or (4) F,14,16,19,15,13,215.
3. Results
3.1 Serological distribution of Legionella species
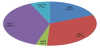
A total of 271 isolates of Legionella were isolated from hospital cooling tower (49%), public buildings cooling tower (24%), public bath (14%), kitchen (8%), tanks (3%) and swimming pool (1.5%). Seventy three percent of the total samples were collected from cooling tower water, and the rest of the samples were collected from hot water. Among the 271 isolates, the 260 L. pneumophila isolates statistically predominated (96%), whereas Legionella species other than L. pneumophila accounted for 4% of the total. Among the 102 sequenced L. pneumophila, the sg 7 strain accounted for 39 (38%), whereas strains sg 3, sg 1, sg 10, and sg 4 accounted for 32%, 19%, 7%, and 4%, respectively (Figure 1).
3.2 Analysis of geographic distribution of Legionella pneumophila
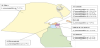
L. pneumophila sg 1 was prevalent in 4 governorates throughout Kuwait, and was represented in Al Asimah (10%), Mubarak Al-Kabeer (7.7%), Al Ahmadi (1.3%) and Al Farwaniyah (2.5%). L. pneumophila sg 3 and sg 10 were represented only in Al Asimah governorate (36.3%) and (6.3%), respectively. L. pneumophila sg 7 was detected in 3 governorates, Al Asimah(17.5%), Hawalli (5%), Al Jahra (5%). L. pneumophila sg 4 was detected in 2 governorates, Al Asimah (3.8%) and Al Ahmadi (1.3%)(Figure 2).
3.3 Legionella pneumophila serotype according to facility type
L. pneumophila sg 7 accounted for 38.2% (39/102) of the total isolates and predominated in facilities such as buildings and cooling towers (hospitals), although not in public baths. The distribution rates of L. pneumophila strains other than sg 7 depended on the facility types; L. pneumophila sg 3 (32.4%) prevailed in public baths, buildings and cooling towers (hospitals), L. pneumophila sg 1 (18.6%) in public baths, buildings and cooling towers (hospitals), and L. pneumophila sg 4 in buildings and cooling towers (hospitals)(3.9%). L. pneumophila sg 10 (6.9%) predominated in in public baths and buildings (Figure 3).
3.4 Legionella pneumophila sero type in cooling tower water and hot water
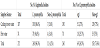
In order to determine whether the distribution of Legionella pneumophila depended on the sample type, the species and sero group distributions were compared between the 72 isolates from the cooling tower and the 30 isolates from hot water (Figure 4). The predominant strain in the cooling tower was L. pneumophila sg 7 accounted for 52.8% of isolates collected from cooling towers. The secondarily dominant strains depended on the sample type was L. pneumophila sg 3 accounted for 66.7% of isolates collected from hot water. In our comparative analysis of distribution between the cooling tower and the hot water, L. pneumophila sg 1 (18% and 20%, respectively), sg 4 (4.2% and 3.3%, respectively) and sg 10 (7% and 6.7%, respectively) were the predominant strain in both sample types.
3.5 Genetic diversity
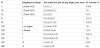
For SBT, among 260 isolates of L. pneumophila, 102 isolates were selected randomly, and these isolates were differentiated by SBT into 11 different sequence types (STs). ST1718 of L. pneumophila sg 7, as the predominant type, accounted for 37.3%, and ST1555 (1%) was found only in one of the cooling tower samples. The profile for the two STs could not be found in the EWGLI SBT database. For L. pneumophila sg 4 (los Angeles), ST1719 (1%) was found only in one sample of hot water, whereas ST1436 (2.9%) of L. pneumophila sg 4 (Portland) was found only in the cooling tower. Both ST profiles could not be found in the EWGLI SBT database. ST154 (1%) of L. pneumophila sg 1 (Oxford/OLDA) was found only in one of the hot water samples, ST 752 (7.8%) only found in cooling tower samples and ST1 (9.8%) was commonly distributed. ST1604 (1%) of L. pneumophila sg 10 was found only in one of the cooling tower samples, ST 1223 (5.9%) was commonly distributed. Both ST profiles could not be found in the EWGLI SBT database. ST93 (12.7%) and ST336 (19.3%) of L. pneumophila sg 3 were commonly distributed (Table 1).
4. Discussion
This study, part of it has been published, is the first study that provides the distribution and genetic diversity of environmental L. pneumophila serotype isolated from water in cooling towers and hot water systems in Kuwait. Among the cooling tower systems and hot water systems, the predominant species, L. pneumophila, accounted for 96.4% and 94.6% of the total isolates, respectively. L. pneumophila have been demonstrated by other studies to be the predominant species in water of cooling systems and hot water systems [3,19,32,33]. The ecology of L.pneumophila serotype was found differed between the water in cooling towers and the hot water samples collected from public facilities. L. pneumophila sg 7 was identified as a major strain (52.8%) in water of cooling towers and L. pneumophila sg 3 as a major strain (66.7%) in the hot water samples. L. pneumophila sg 1, compared to only 27% of the isolates collected from water in cooling towers and 25.7% of the isolates collected from hot water (Table 2). The results of this study differed from those reported in previous studies of public facilities. Specifically to L. pneumophila strain, sg 1 was the most frequently detected strain in cooling towers and hot water [3,19,32,33].
In the comparative analysis of the SBT distribution of the isolates according to sample type, ST1718 was the predominant type in the isolates from the cooling tower water and ST336 was the dominant type in the hot water samples. However, STs 752, 1436, 1555, and 1604 were found only in isolates from the cooling tower water, and STs 154 and 1719 were found only in the hot water isolates(Figure 4).The predominant profile for sg1 in this study was ST1 (1, 4, 3, 1, 1, 1), which is broadly distributed throughout the world [22,34,35,36]. STs 1719, 1436, 1718, 1555, 1223, 1604 were not detected in the EWGLI SBT database or in any other studies. Our results are similar to those observed by Kozak et al. (2009) [37], who reported that 58% of the STs found were unique to the United States.
5. Conclusion
In conclusion, the results shown that the comparative populations of environmental isolates of L.pneumophila stains isolated from public facilities varied according to the types of facility as well as the geographic allocations of the facilities. Also, the results revealed several unique allelic profiles of STs and showed that ST1718 of L. pneumophila sg 7 was the prevalent sequence type in Kuwait. This study highlight the significance of understanding the epidemiology and ecology of L. pneumophila strain from public facilities in terms of public health and provide useful information for future epidemiological investigation of local and regional outbreaks of LD. Thus, routine monitoring of environmental water for Legionella species is an auxiliary implement to reduce the bacterial contamination of water systems and to assist the development of a more active prevention strategy. Further study will require the focus on correlation analysis by clustering between environmental and clinical isolates of Legionella strains.
Competing Interests
The author declare that there is no competing interests regarding the publication of this article.