1. Introduction
The vascular atherosclerotic process is the leading cause of cardiovascular (CV) diseases. In order to reduce CV disease’s morbidity and mortality in the developed and, now more frequently, in the developing countries, efforts are undertaken to control the risk factors like smoke, obesity, sedentary life, dyslipidemias, hypertension and diabetes,that underlie CV diseases. On the other side huge work has been done to make it possible to correctly diagnose the presence of the atherosclerotic calcified and non-calcified plaques, that cause coronary artery vessel lumen reduction,which leadto myocardial ischemia, asymptomatic myocardial dysfunction, myocardial infarction, life threatening arrhythmias and sudden death. In more than a half-century’s experience invasive coronary angiography (ICA), particularly with its tools like intravascular ultrasound (IVUS), intracoronary Doppler, fractional flow reserveand optical coherence tomography,proved to be the “gold standard” technique to evaluate coronary arteries and,more importantly,to be extremely reliable in the diagnostical processes of coronary artery disease (CAD) patients. The introduction in the first years of the third millennium of multidetector row systems in the field of cardiac computed tomography (CCT) has made imaging of the heart and in particular of epicardial coronary arteries feasible [1]. Nowadays CCT is used as a complementary test to ICA. CCT has a good accuracy compared to ICA, but more interestingly it provides its information non-invasively [1].
Besides the atherosclerotic CAD lesions there are other nonatherosclerotic coronary artery vessel lumen reductions, although their prevalence is less common. These vessel lumen reductions arerelated to prolonged coronary artery spasm, hypertrophic cardiomyopathy, vasculitis and congenital coronary anomalies. Clinically these pathologies are evaluated with the same diagnostic tests used to study the atherosclerotic CAD lesions: electrocardiogram, exercise stress test, trans-thoracic, trans-esophageal and stress echocardiography, myocardial perfusion imaging, magnetic resonance, ICA and CCT. In particular non-atherosclerotic CAD settings CCT can be even considered the “real gold standard” techniqueinstead of ICA [2,3]. Congenital coronary anomalies are a heterogeneous group of diseasesand occur in 0.17% of the autopsy cases [4].
In this paper we will describe an illustrative case of a patient,suspected to have a CAD, but in whom with CCT we diagnosed a myocardial bridge (MB). We will provide a mini-review of the literature on MBs. We will describe the diagnostic procedures as well the therapeutic alternatives with particular attention to those studies performed with CCT.
2. Illustrative Case
The patient of this case report is a 53 years old active female.She was doing fine till April 2015 when she began to suffer of left sided chest pain, associated with dyspnoea and aggravated by exercise and anxiety. She did not have a family history of relevant diseases, no past history of allergy, surgical intervention, diabetes, hypertension, and did not take any kind of medications. Basal physical examination, blood pressure(120/80 mmHg) and blood tests were within normal limits. No abnormalities were found in the electrocardiogram; her heart rate was 68 beats perminute. Chest X-ray was also normal. However, since chest pain becamerecurrent and of sudden onset, she underwent a maximal treadmill stress test, which showed ST depression (1.5 mm) and negative T wave in the inferolateral leads. The patient was then sent to perform a dipyridamole stress echocardiography, which showed an improvementof the basal hypokinesia of the medial and apical segments of the septum and the anterior wall. An anatomical evaluation of the coronary tree was therefore needed and she was sent to our CCT laboratory.
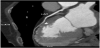
CCT was performed with 64-slice Brilliance scanner (Philips Healthcare) by administering 100 ml of iodinated contrast medium (Iomeron 400 mg/dl, Bracco Imaging, Italy) followed by 50 ml of saline solution at a rate of 5 ml/s through 18 gauge cannula placed in the antecubital vein. Images were acquired during a 10 to 15 second breath-hold, with retrospective electrocardiographic gating. After data acquisition on dedicated workstation (Philips Extended Brilliance Workspace; Philips Medical Systems) images were reconstructed especially at the 75% of the RR interval, with a thickness of 0.6 mm and increment of 0.3 mm. To reduce patient’s heart rate, 30 minutes before the test she was given 50 mg of Metoprolol per os. CCT angiograms provided images, which were judged of good quality and both right and left coronary arteries were correctly visualized (Figure 1).
Right coronary artery (RCA) was the dominant vessel; no lesions were seen in the RCA and in the posterior descending coronary artery.
No lesions were seen in the left main, circumflex coronary artery, diagonal or marginal branches. Also the left anterior descending artery (LAD) was free from lesions; however in the mid portion of the LAD the vessel dipped into the heart muscle to come back out again after a tunnelled segment of 15 mm (Figure 2, Figure 3 and Figure 4).
In this case the CCT was very useful as it provided a comprehensive anatomical view of the coronary tree and indicated the presence of a MB in the mid portion of the LAD. Beta-blocker treatment was introduced with symptoms’remittance. At the 3 months follow up the patient was doing fine.
3. Discussion
Congenital coronary artery anomalies in the majority of cases lack clinical significance and are merely epiphenomena found accidentally during necropsies, while performing invasive or non-invasive coronary angiography or during surgical interventions. However in some cases they may be responsible for chest discomfort, malignant arrhythmias, fatal or non-fatal acute myocardial infarction, ventricular septum rupture, myocardial stunning, paroxysmal atrio-ventricular block, syncope and sudden death [5]. In particular 19% of sudden deaths in young athletes were due to coronary artery anomalies [6].
Myocardial bridges (MBs) are the second most common type of coronary congenital anomalies and are inborn coronary anomalies, considered normal variants of intrinsic coronary arterial anatomy with an intramural course [4]. In humans coronary arteries and their main branches have an epicardial course running over the cardiac musculature. In the presence of a MB a portion of one coronary artery or more dips into and underneath the heart muscle to come back out again in the majority of the cases.
In 1951 Geiringer gave a comprehensive post-mortem description of the presence of a MB overlaying the LAD [7]. The real incidence of this entity is unknown and varies according to the procedure used to study it: MBs are rare in patients referred for cardiac surgery (0.2- 0.3%) orICA (0.4-4.9%) while they are very frequent during autopsy (5.4-85.7 %) [8]. According to some Authors superficial MBs may not be exclusively congenital in origin, but may result from adulthood disease processes that partially cover the artery with fibro-fatty connective tissue [8].
Although autopsy studies did not demonstrate any difference in the frequency of MBs by age or sex, angiographic studies indicate that males have a higher incidence and longer MBs probably owing to a higher musculature of the body in respect to females [9,10]. MBs seem to be more present in orthotopic heart transplantation patients [11] and in the paediatric subpopulation with hypertrophic cardiomyopathy [12].
A milestone work in studying MBs is that of Ferreira et al. who found a MB in 50 of the 90 hearts studied (55.6%), mainly on LAD. These Authors distinguished twotypes of MBs: a superficial type (75% of cases) and a deep type (25% of cases) and speculated that the vessel may be more distorted and compressed in the deep ones [13].
Konen et al. in their analysis using CCT described three types of MBs. Besidesthe superficial (29% of cases) and deep (41% of cases) types these Authors characterized a third one called “right ventricular type” (29% of cases). In thistype the LAD “disappears” and is visible only in the axial imageswhere it has a course near the right ventricular wall. This “right ventricular type” seems to be more potentially pathologic and more difficult to be treated surgically [14].
MBs can be also classified depending on the length and the number (one or more) of MBs or according to the coronary artery and the segment of the coronary artery involved. The majority of MBs are in the mid portion of the LAD, like in our illustrative case. However MBs have been also found over the proximal and distal segments of the LAD and over the diagonal branches. Bridging of the circumflex or the RCA or one of their branches is not so common [8,9]. In the presence of two parallel LADs one of them frequently takes an intramural course [8].
ICA is still considered the gold standard technique to study in vivo MBs and chest pain is the most common reason for angiography. Porstmann and Iwing in 1960 were the first to report,in a 19-year-old patient, the radiological appearance of transient stenosis in a segment of the LAD during systole [15]. The typical angiographic finding of a MB is a systolic narrowing of an epicardial artery, known also as a “milking effect” phenomenon induced by systolic compression of the tunnelled segment. The milking effect is evaluated as grade I when the narrowing is less than 50%, grade II when it is between 50 and 75%, and grade III when it is greater than 75% [16]. Another angiographic finding is the presence of the “step down-step up” appearance, namely, a significant tortuosity of the segment beneath the MB at the entrance (step down) and the exit (step up) sites [10,16]. However, in the presence of a MB also the diastole is compromised. In fact measurements in patients with MB have shown a persistent diastolic diameter reduction enduring mid diastole [16].
The wide variation at angiography of MB cases (from 0.5 to 33%) may be attributable to technologic advances in cine-angiography, to the orientation of the coronary artery and myocardial fibres, to the state of myocardial contractility, to the fact that small and thin bridges cause little compression badly detectable during angiography, if the study was retrospectively reviewed for the specificpurpose of assessing the frequency of MBs, to different population selection and sample size and probably also to ethnicity [10].
The performance of ICA increased with the introduction of important tools such as IVUS, that for the first time visualized, in vivo, both vessel lumen and walls. IVUS studies confirmed the autopsy studies that recognized “sparing” of bridged segments from atherosclerotic lesions [17]. This is because the intima of the tunnelled segment is significantly thinner than that of the proximal segment, and contains more contractile subtype of smooth muscle cells thought to be negatively associated with the development of atherosclerotic lesions, while foam cells, an important component of atherosclerosis, are missing [18]. In the bridged vessel wall also the expression of known vasoactive agents (endothelial nitric oxide synthase, endothelin-1, and angiotensin-converting enzyme) is reduced [19]. Conversely, the vessel segment proximal to the bridge ap¬pears to develop atherosclerosis at increased rates with higher expression of vasoactive agents. This explains the increased plaque formation prox¬imal to the tunnelled segment. A characteristic and highly specific observation obtained with IVUS in patients with MBs is the“half moon phenomenon”, an echolucent area surrounding only the tunnelled segments. In the presence of the “half-moon phenomenon” the milking effect can be induced by intracoronary provocation tests, such as intracoronary nitroglycerin injection,even if the bridge was previously angiographically undetectable [17]. Ultrasound pullback studies performed with pressure-wire revealed the characteristic flow pattern: “fingertip phenomenon” or “spike-and-dome pattern”, which is present in most of patients with MBs. This flow pattern described by Ge et al. [20] consists in a sharp acceleration of the flow in early diastole followed by an immediate marked deceleration and a mid to late diastolic pressure plateau. The Authors explain this flow pattern as an increase in the pressure gradient in the early diastole as a result of a reduced distal coronary resistance while there is a delay in the relaxation of the myocardial fibres. The subsequent sharp deceleration in the coronary flow velocity results from the relaxation of the myocardial fibres and an increase in the vascular lumen. After the release of the compression, the lumen of the bridge segment remains unchanged in the second half of the diastole and this corresponds to the plateau of the flow pattern at this phase. In deep MBs, rapid diastolic forward flow may be preceded by end-systolic flow inversion as a result of systolic squeezing of the bridge segment. The consequence of these phenomena is that in the segment proximal to the MB the pressure can become even higher than that in the aorta. At the entrance of the MB the high wall stress and disturbance in blood flow promote atherosclerosis [20]. Finally in subjects with MBs the coronary flow reserves, defined as the ratio of mean flow velocity achieved at peak hyperemia obtained after intracoronary injection of papaverine or adenosine to mean resting flow velocity, is frequently reduced (2.0-2.6), values below 3.0, which is regarded as the lower normal [16].
However ICA provides only a few 2D view images of the coronary arteries. With ICA it is not always easy to selectively engage the anomalous coronary vessel and it is not always possible to have a clear idea of the course of the coronary vessels within the heart and the relationship between the coronary vessels and the surrounding structures. For these reasons the introduction in the cardiac arena of the CCT was particularly useful to study coronary artery anomalies because it provides an unlimited number of 2D and 3D images of the single vessel [1]. This information is very useful to the surgeon: a preoperative diagnosis of MBs on CCT may helpin planning the surgery strategy (midsternotomyor a minimally invasive approach) especially in case of extensive and deep MB in order to avoid, for example, accidental opening of the right ventricle during dissection of intramuscular LAD [14].
In the recent American Appropriate Use Criteria Task Force for CCT, the use of CCT in the “assessment of anomalies of coronary arterial and other thoracic arteriovenous vessels” was pointed to be most appropriate (i.e. the test is acceptable and considered a reasonable approach to study the disease and its expected incremental information, combined with clinicaljudgment exceeds the expected negative consequences by a sufficiently wide margin)with a score of 9 out of 9 [2].
An important limit of CCT in studying patients with MB is related to the fact that CCT analysis are mainly performed with images reconstructed during diastole (70-80% of the cardiac cycle) when there is the maximal vasodilatation and minimal motion artefacts. Conversely maximal lumen narrowing of the MB is during the systolic phase (30-40% of cardiac cycle) where usually there are more motion artefacts. To better evaluate patient’s MB it is therefore important to analyse the whole cardiac cycle. Most recent scanners have high temporal resolution enabling the visualization of the vessel lumen during most of the cardiac cycle,and thus permitting the observation of the milking effect in the 4-dimensional reconstruction [21].
Since the introduction of CCT many papers have been publishedshowing the feasibility of CCT in evaluating patients with MB [22].
In CCT papers MB is usually defined asthe existence of tissues exhibiting soft tissue density (same contrast enhancement as myocardial tissue) covering part of the vessel [23]. The length of the MB is usually defined as the distance fromthe entrance to the exit of the tunnelled artery measured by curved multiplanar reconstructed images(i.e. parallel to the course of the vessel) [23]. The depth of the MB, measured in an axial image (i.e. perpendicular to the course of the vessel),in the majority of the papers is defined as the thickness of the deepest part from the surface of the covering myocardial tissue to the tunnelled artery [23]. Another classification divided MB in complete or incomplete: in the complete type it is possible to demonstrate the continuity of myocardium over the tunnelled segment [24].
The prevalence of MB in CCT studies increased progressively with the introduction of more modern scanners approaching values found in autopsy studies, which should be considered the ultimate gold standard method to analyse MBs [22].
The wide variation in frequency of the clinical presentation of MBs cases indicates that many MBs do not produce symptoms. Subjects may become symptomatic after the third decade of life unless MBs are associated with precipitating factors (i.e. high heart rate, increased myocardial contractility, hypertrophic cardiomyopathy, decreased peripheral vascular resistance) [1,8].
MBs cause blood flow alteration, which may induce chronic and or acute transient myocardial ischemia and be responsible for life threatening arrhythmias, such as ventricular fibrillation. In fact many of the analysed hearts withMBs were from subjects who died of sudden death [6,25].
A fairly large percentage of subjects with MBs may have concomitant atherosclerotic, muscular, or valvular heart diseases, which may independently affect the clinical outcome as well as the treatment strategy [8]. Typically, the MB patients are 5 to 10 years younger than those with symptomatic CAD. Typical angina is present in 55% to 70% of the cases, and atypical angina is often reported in association with rest angina. The co-presence of MBs with atherosclerotic CAD should be taken into account when it is not possible to detect a culprit lesion in symptomatic patients. This is the case of the patient of our illustrative case. Although MBs have excellent prognosis even in patients with ≥ 50% systolic compression, early diagnosis and treatment are important due to their possible complications [14].
In case treatment in symptomatic patients is needed firstline therapy should primarily include beta-blockers and if betablockers are not tolerated, calcium channel blockers with negative chronotropic effect,as they reduce the compression by the muscular band and slow the heart rate by prolonging diastole. As atherosclerotic changes proximal to the tunnelled segment are frequently found antiplatelet drugs and statins may be also considered as a preventive measure.Stent implantation is no more considered a therapeutic option because the compression of the vessel wall against the stent may cause stent fracture or vessel wall damage and in-stent stenosis. Surgical treatment of MBs is another option. By-pass surgery and in particular left internal mammary artery anastomosis to the LAD has been advocated especially if the MB is located in the proximal LAD. However,myotomy, although it presents some problems if the bridge is long and deep, is considered the best non-medical option [8,26].
4. Conclusion
This illustrative case and the review of the literature show that CCT in nowadays the in vivo “gold standard” technique to evaluate patients with MB. However to better understand the real usefulness of CCT in this particular field, further multi-centric interdisciplinary studies must be performed, to link the morphological with the clinical information especially in those patients who have MB and normal coronary arteries or coronary arteries with no culprit atherosclerotic lesions, but who may be at risk for CV morbidity or mortality.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Acknowledgments
We gratefully thank Dr. Haifa Alsakkaf for the assistance with the manuscript and the radiology team of Fanfani Clinical Research Institute for their collaboration.