1. Background
Epidermolysis Bullosa (EB) is a rare genetic disease, with an overall prevalence of about 1:17,000 births, characterized by detachment of the epidermal and dermal layers, spontaneous or resulting from minimal traction or rubbing of the skin, which can occur from birth throughout the lifespan.
It can manifest with different degrees of severity; clinical manifestations can range from localized blisters on the hands and feet to generalized lesions of the skin and oral cavity, and can lead to severe damage to internal organs. They can appear at birth or shortly after; in some localized forms, the lesions can also appear in late childhood or early adulthood [1-3].
Each EB subtype results from mutations within genes that code for different proteins, each of which is involved in maintaining the structural stability of the keratinocytes or in the adhesion of the keratinocytes to the underlying dermis. Sixteen different genes have been identified whose mutation cause EB; the genetic mutation involves the alteration of the formation or functioning of proteins responsible for anchoring the epidermis and dermis; consequently, this anchoring may occur correctly and, even in the case of minimal mechanical traction, the formation of bullous lesions occurs [1,4,5].
The current international classification distinguishes EBs into four major types, i.e. EB simplex (EBS), Junctional EB (JEB), Dystrophic EB (DEB), Kindler’s Syndrome (SK) (Table 1).
A complete medical history is essential to correctly diagnose EB, including family and personal medical history, analysis of cutaneous and extracutaneous manifestations and identification of the time of onset of symptoms.
If a suspected skin fragility is identified, abnormalities in the proteins that make up the skin can be hypothesized. Laboratory investigations include blood chemistry, microbiological, and immunological analyses on skin biopsies.
To distinguish the different types of EB, it is necessary to identify in the epidermal-dermal layers the level at which the bullous lesions develop, with antigenic mapping by immunofluorescence which is performed by skin biopsy, and trasmission electron microscopy [3,4].
Genetic analysis therefore allows to confirm the type of EB. In the neonatal period, especially in the absence of a positive family history of blistering or if the clinical laboratory findings are atypical for EB, prenatal herpes simplex infection should be considered.
Unfortunately, there is currently no cure for EB. The only intervention options are those of wound management, prevention of associated infections and chronic pain management. There are no guidelines that apply to all subtypes of EB, because each variant has a different type of lesion, which must be treated specifically [1,6,7].
The prognosis for patients with EB is highly variable based on the disease subtype. Most patients, especially those with EB Simplex and Dystrophic EB, have normal life expectancies, although they often need health care to manage injuries and possible complications associated. On the other hand, patients with Junctional EB have a higher incidence of mortality during the first years of life. Other variants of EB, such as recessive EB, expose the affected individual to a greater risk of death at a young age or adulthood from metastic squamous cell carcinoma [1,8,9].
2. Objective
Since newborns with EB have to be handled with extreme care and delicacy, this work aims to report the management of a newborn with EB and to identify useful information for optimal care for these infants, focusing primarily on nursing care.
3. Materials and Methods
We report the overall management of a newborn with EB admitted to the Regina Margherita Children’s Hospital, focusing the analysis on patient’s nursing care. The literature review of the literature was conducted by consulting articles and evidences from the Pubmed, Cinahl and Uptodate databases, using the following keywords: “Epidermolysis Bullosa (Mesh)”, “Newborn (Mesh)”, “Nursing”, “Wound care”, “Skin”, “Pain”, “Itch”. The relevant articles on EB have been used to have a general overview of the disease management, on the most correct one, focusing on the most important nursing issue for the optimal care of these extremely fragile infants.
4. Case Report
We report the real-life experience of managing a newborn with EB, a rare and severe disease with a complex care management.
In June 2020 the newborn was admitted to the Subintensive of Early Childhood (SAPI) department of the Regina Margherita Children’s Hospital, with a clinical picture attributable to Junctional EB, subsequently confirmed by the results of histological, immunohistochemical and molecular investigations.
At birth, the child had bullous lesions on the hands and nail dystrophy and, upon admission to SAPI, lesions on the back of the hands, in the perionichium of the big toe of the left foot and of the second finger of the right hand, small erosions on the right thigh and on the left flank and in oral mucosa mainly at the lower gingival level, in the middle and in the hard palate.
Due to the severity of the child’s condition and the high risk of infection, from the entrance to the ward, the child was placed in contact isolation, in a single room where the health staff accessed using the appropriate devices. The room was equipped with two dedicated trolleys, one with all the material necessary for daily medication and another for the preparation of therapies. Before coming into contact with the child and before dressing personal protective equipment (PPE), which involves non-sterile gloves and surgical mask, the hands were sanitized with antiseptic soap or hydro-alcoholic gel.
After carrying out the various procedures, before leaving the patient’s room, the used devices were disposed in the container for hazardous medical waste with infectious risk; then a new hand sanitization followed (Table 2).
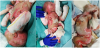
At the time of admission, the child had skin lesions on the entire body surface, mainly affecting the feet, legs, thighs, buttocks, scrotum, trunk, elbows, arms, hands, neck, mouth and scalp, characterized by the frequent onset of sero-haemorrhagic bubbles which, if left treated, evolved into thick crusts with granulation content (Figure 1).
The blisters originated both from the rubbing of the affected areas and spontaneously. The wound dressing was initially managed by the surgical team, usually involved in the management of burn wounds, and by the dermatological team; subsequently electively by the latter.
The dressing was performed in the room by the dermatologist and two nurses, once a day, in the morning; the time spent ranged from 30 to 45 minutes, during which pain was constantly assessed to eventually modulate the analgesic therapy.
Due to the high loss of fluids from the skin and the risk of causing further injuries, the child was kept at rest on an anti-bedsore mattress and covered with removable sterile sheets, renewed whenever they were soaked, and every 3-4 hours when the child posture was changed.
The first dressings involved touches with Eosin, and topical Rifamycin, used for the treatment of infected wounds, on suppurating lesions, in order to keep them dry; the same were subsequently treated with a spray dressing containing hyaluronic acid and metallic silver, to allow healing.
They have been covered with vaseline gauze, which prevents adherence to the wounds and allows a non-traumatic dressing change, thus ensuring the natural healing process.
At a later stage, on the advice of the dermatologist consultant, the dressing was modified, using touches with Eosin and the bandage with, the non-adherent Adaptic dressing, on the limb lesions, while the trunk lesions were covered with Mepilex, a soft foam dressing which absorbs exudate and prevents rubbing.
Aureomycin 3% ointment was sometimes applied to treat areas with large crusts that could not be removed.
For such clinical picture from birth a central venous catheter (CVC) was placed for any repeated blood sampling, the administration of drugs and eventual parenteral nutrition. Intravenous antibiotic and antalgic therapies were therefore set up, both essential to counteract the loss of fluids due to the extent of skin lesions and the very high risk of infection and for the chronic pain that afflicted the child due to the wounds. The child also had a urinary catheter to ensure diuresis, as the genital mucosa was at risk for urethral meatus stenosis due to potential scarring of the lesions, resulting in urinary retention and possible outcome for hydronephrosis. Nasogastric tube was then placed, with the aim of eliminating unwanted gastric contents and eventually administering nutrients, the newborn was initially fed enterally through a nasogastric tube; subsequently, this device was removed as the child could no longer tolerate feeding, as the lesions also involved the gastrointestinal tract; the infant was then fed through Total Parenteral Nutrition (TPN).
Due to the vast extent of the lesions on the child’s skin surface, it was necessary to administer analgesic therapy to reduce the perception of pain as much as possible. At the Regina Margherita Children Hospital, different scales are used based on the age of the child and his ability to communicate with the operators; the FLACC Scale (Face, Legs, Activity, Cry and Consolability), based on a behavioral observation of the newborn and the child aged 0-3 years to determine the presence of pain [10-12], was choosen to monitor pain in the baby. Pain was assessed by the nursing staff at least once a day or for each shift and also before and after the execution of painful interventions, during the administration of analgesic drugs or after their suspension and upon reporting the presence of pain by the patient or caregivers.
After having correctly assessed the pain, it was necessary to administer a proper analgesic therapy. The non-pharmacological treatment of pain was taken into consideration as an integral part of the analgesic therapy; it was always performed before the use of drug therapy and also in combination with it. The advantages found in treating pediatric patients with non-pharmacological therapy are different: it is free of side effects, easily implemented, and does not have a great cost.
The non-pharmacological treatments were also noted on the health records.
During the hospital stay, the prevention and treatment of pain was set up with the use of multiple drugs combined with each other, modulated and dosed according to the pain detected through the FLACC scale. Morphine and clonidine were used in continuous, or as administration of bolus doses of morphine initially enterally, then intravenously, before wound dressing and as needed.
The analgesic therapy was set up following the prescriptions of the clinician expert in palliative medicine, part of the working group “Hospital without pain”, a project approved by the WHO and used in all western countries, which has as its main objective to create cultural and operational tools to fight pain in chronic pathologies in which pain has lost its alarm bell purpose and becomes a real pathology.
5. Evidences from the Literature
Starting from the evidence in the main databases, some useful information has been selected for a good nursing management of the patient with EB, which includes pain management, skin management, itching management and finally the malabsorption of nutrients and complications associated with it.
5.1 Pain management
One of the great problems of patients with EB is the presence of both chronic and acute pain, caused by the continuous formation of lesions to the skin and mucous membranes. Thus, it is essential to ensure the patient a good analgesic coverage to minimize suffering and any other problems associated with the perception of pain, such as anxiety and depression. In general, a bio-psycho-social approach is useful, which uses medical, psychological and social interventions for pain management [9,13].
First of all, it is important to regularly carry out a proper pain assessment, in order to implement analgesic interventions, and subsequently to evaluate the analgesic effectiveness of the intervention. In the newborn, the FLACC Scale (Face, Legs, Arms, Cry, Consolability) can be used, in the school-age child, the Wong- Baker scale, composed of six faces, while in children over the age of eight, the use of the numerical rating scale is effective, such as the visual analogue scale, which assigns a numerical value to pain from 0 to 10, where 10 indicates the worst pain ever felt [10-12].
Psychological interventions have been shown to play an important role in reducing pain intensity and related anxiety and functional disability. These treatments can be used in painful procedures, such as wound dressings, or in chronic pain conditions. An example of psychological therapy is cognitive-behavioral therapy (CBT), that focuses on changing catastrophic thinking and negative emotions related to pain and lifestyle modification to adopt behaviors that can produce well-being, despite the chronic pain. Alternatively, techniques that include muscle relaxation, diaphragmatic breathing and imagination, coping strategies that use distraction and activation of the parasympathetic nervous system can be used. Although these interventions are often patient-centered, CBT can also include caregivers, to reduce their distress and improve environmental factors to reduce patient’s stress [2,9,13,14]. Pharmacological treatment of pain in patients with EB is not universal; according to the analyzed guidelines, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), Paracetamol, Tramadol and opioids can be used with positive effects. To ensure a baseline level of comfort, many patients with severe EB subtypes use long-acting opioids, but there are no guidelines that indicate long-acting opioids ad preferable to the use of intermittent ones [13]. Methadone is also sometimes used in the pain relief of the patient with EB, but adequate electrocardiographic monitoring is necessary, for the increased risk of QT prolongation. When wounds are particularly extensive, much of the perceived pain is neurophatic and therefore there is evidence to support the use of gabapentin and tricyclic antidepressants, even with tricyclic use, special attention should be paid to patients at risk of developing cardiomyopathy, for example patients with recessive DEB, as this drug also may be responsible for QT prolongation [13].
Regarding topical therapy, in addition to implementing all necessary measures to reduce trauma during dressing replacement, local anesthetics can be used on intact skin, but cannot be recommended for wounds, as the extent of absorption of lidocaine is not known. Lidocaine injection with a small gauge needle is considered a standard approach for superficial analgesia [13].
5.2 Skin management
While taking all necessary preventive measures, it is very difficult to prevent the formation of lesions in patients with EB throughout life. This is why it is important to adopt useful behaviors to limit the onset of injuries and learn how to manage them optimally; injuries are dangerous entry points for pathogens that can cause systemic infections, and limiting the chronic painful stimulus associated with them is essential to ensure the best possible quality of life in EB.
The extent and nature of the lesions is highly variable depending on the EB subtype, the frequency of treatment and the need for assistance are therefore also variable, as there is no universal cure for all lesions, as each is different based on subtype and location [2,15].
Numerous systemic and local factors can influence wound healing and, among the systemic factors, nutritional status is primarily involved. If the body is well nourished, the metabolic processes of the body will function properly, including the healing of injuries; in the subject with EB, often the supply of nutrients is not sufficient to satisfy the metabolic needs of the organism, and this negatively affects the management of the skin [1,16].
The most important nutritional components for the healing of skin lesions are albumin, which regulates osmotic fluid, carbohydrates, which provide energy to cells allowing them to save proteins, vitamin A, which promotes the synthesis of glycoproteins, increasing regeneration tissue, Vitamin E which has antioxidant properties and maintains the integrity of the cell membrane and Vitamin K for its function in blood clotting and healing processes [1,16].
Among local factors, local factors, maceration of the periwound skin due to excess fluid leaking from the lesion, recurrent trauma, if the injury is in a site subject to repeated rubbing and local infection are those to be considered mainly. In general, it is good to ensure good skin hygiene, dry the skin by tamponade and not by rubbing, and keep it dry to avoid maceration; in case of dry skin, use emollient creams to keep it elastic [1,17,18].
In the case of localized forms of EB, such as simplex EB or dominant dystrophic EB, where lesions affect only certain areas of the body and not continuously, the dressing can be replaced more quickly than in generalized forms.
To perform a correct wound dressing in the child with EB many step should be considered. To carry out the dressing, first a place must be set up; in the case of newborns, the changing table can be used as a dressing table. It is also important to create a warm environment as the newborn has a very labile thermoregulation system, so low temperatures could open wounds causing more pain. To reduce the execution time, once the site to perform the dressing has been identified, the material to be used can be prepared, such as ointments, non-adherent dressing, gauze, elastic bandages. It is useful to open the packages, cut gauze and bandages already, to size and prepare a waste container.
Subsequently, a correct disinfection of the hands must be carried out; due to the high risk of infection of the lesion, it is important that all who take part to the procedure carry out a thorough antiseptic hand washing; any rings or bracelets must be removed before disinfection.
After disinfection of the hands, it is necessary to wear PPEs, which include the surgical mask, visor, disposable qown and non-sterile disposable gloves to remove the dressing. Once old dressings are removed, the gloves are replaced with a sterile disposable pair [1].
Then, the old dressing should be gently removed; to moisten it, if it remains adherent to the underlying wound, a sterile sodium chloride solution could be used, by sponging the lesion [1,18].
Once the bandages have been removed, the state of the lesion has to be observe; to get a general picture of the skin lesions and their evolution, it is advisable to report the location, size and appearance of each of them in the clinical documentation, so as to be able to follow the course [1].
In particular, the dimensions, with maximum length and width of the lesion, the depth, if there is superficial abrasion or blisters or deep injury with or without weakining of the adjacent tissues should be noted.
The presence of exudate should be considered, quantitatively, if absent, scarce, moderate or abundant, and qualitately, if serous, purulent or bloody, as well as the appearance of the lesion, whether fibrinous, granural, necrotic, and of the periwound skin, if normal, macerated, edematous. Finally, the presence or absence of pain perception should be noted [2].
Each time the dressing is replaced, the lesion must be cleansed to promote a decrease in the bacterial load, which can otherwise turn into an infection. With cleansing, metabolic debris, old dressing residures and exudate of non-viable tissue are eliminated. Sodium chloride 0.9% solution can be used to allow adequate irrigation of the lesion. Cleaning is not intended to sterilize the wound, so it is not recommended to use antiseptics. If the lesion requires disinfection, the preferred antiseptic is chlorhexidine gluconate in 0.05% aqueous solution [1].
After cleansing, if large bubbles (greater than 5 mm) are present, it is advisable to puncture them with a sterile large-caliber needle or scalpel.
The fluid inside the bubble must then be drained and the residual skin removed during the subsequent dressing. Conversely, if the lesions are small in size or are located within the oral cavity, it is not recommended to puncture them [1,19].
In general, a drying cream is used for moist wounds, while moisturizers or ointments are used for dry wounds. In more advanced wounds, debridement is also useful, with removal of devitalized or contaminated tissue, to prevent the formation of infections and accelerate the healing process. In case of multiple and necrotic lesions, debridement should be performed in the operating room with adequate analgesia [1,19].
Once the application of ointments to the wound is complete, a new dressing will be applied, without using adhesive dressings which tend to tear the skin when removed. The dressing should generally be done through several steps, first involving a non-adherent protective dressing, such as a viscose fiber gauze impregnated with gel, petroleum jelly or paraffin, which lies between the wound bed and a secondary dressing to reduce grip. This can be used to dress superficial wounds with little exudation; alternatively, when there is a medium-abundant exudate or when the periwound skin is perishable, polyurethane semipermeable foams can be used. Gauzes can be applied to the direct dressing to absorb any secretions produced by the wound and at the same time provide protection. To avoid the dislocation of the gauze, elastic bandages can be used, being careful not to over-tighten and cause a tourniquet effect [1,18].
Once the operation is completed, the waste of the used packaging and all previous medications are disposed of, to avoid the spread of the bacteria present in these. Once all the material has been removed, the surface used for the dressing is cleaned with disinfectant. The continuous degradation and regeneration of the skin leads to an increased risk of developing skin cancer; in particular, squamous cell carcinoma is a malignant skin tumor, which originates from the uncontrolled proliferation of squamous cells in the epidermis; in EB patients, mainly in subjects with recessive EB, this tends to develop even more rapidly and metastasize than in the general population, with an average age at diagnosis around 36 years, thus regular monitoring in this type of patients is mandatory [8,20].
5.3 Management of itching
Patients with EB can often experience a chronic itchy sensation in the lesions and periwound skin that prompts them to scratch or rub to relieve discomfort, resulting in skin irritation reactions. The etiology of itching is still unclear; among the receptors on the skin, some are responsible for the perception of heat, pressure and pain and it has long been believed that the receptors that allow pain to be perceived also have a role in the perception of itching, although to date there is no scientific evidence in this regards [21,22].Often the cause of itching is dermatitis; the chemical messengers of the inflammatory cells, especially histamine, stimulate the itching points of perception. In patients with EB, itchiness is mainly caused by wound healing, in the initial regression phase. It is thus necessary to try to limit the sensation of itching as much as possible, through specific treatments, to ensure a better quality of life and reduce the appearance of further lesions due to the reflex action of the scratch.
Several approaches can be used to reduce itching, such as behavioral therapies, topical treatments or systemic treatments. In general, it is recommended to keep the skin weel hydrated by taking an adequate amount of water, bathing in warm water and moisturizing the skin with lotions or moisturizers; it is advisable to avoid the use of gel or alcoholic solutions for friction. It is recommended to use clothing made from non-irritating materials, such as cotton and silk, and to follow the same principle for bed linen.
Keeping fingernails short can helpful limit scratching. As a topical therapy, there is a wide range of products useful for limiting the itching sensation; in general, it is advisable to use creams or lotions to keep the skin well hydrated, in different compositions based on the type of lesions. Those containing zinc or urea are often indicated.
Finally, as pharmacological therapy, when behavioral and topical therapy is not sufficient to ensure a good quality of life, systemic drugs can be used to reduce itching, for example antihistamines, or short-term treatments based on cortisones [21,22].
5.4 Malabsorption and associated complications
In some EB subtypes the lesions can also be localized in the mucosa of the gastrointestinal tract, with important consequences in the nutritional status. Different complications can develop, depending on the site of the bullous lesions; if chronically present at esophagus, an esophageal stricture may develop, leading to dysphagia, with impaired swallowing, a condition that together, with the pain caused by eventual lesions in the oral cavity, will lead to a decreased intake of nutrients [3,16,23].
It is noteworthy that often the patient with EB takes a quantity of nutrients lower than the protein-caloric requirement; moreover, in these subjects the caloric requirement is increased due to the accelerated skin turnover and chronic loss of blood and proteins caused by the bleeding of lesions in the gastrointestinal tract.
Further serious complications include the reduction of iron, folate and vitamin B12, which contribute to develop anemia, and that of carnitine and selenium, which play a role in inducing cardiomyopathy; insufficient intake of fluid and fiber then causes constipation.
All this results in a delay in growth, bone mass and hormonal dysregulation [3,23,25].
To prevent prolonged malnutrition and its associated complications, early nutritional intervention from the neonatal age. If attempts to increase an autonomous oral intake are not adequately effective and the clinical condition indicates prolonged malnutrition or general worsening, it may be necessary to place aids to administer enteral nutrition, such as nasogastric tube or gastrostomy. In the case of compromised digestive mucosa TPN should be considered [3,16,23]. However, enteral nutrition should be preferred, as it has greater advantages in maintaining the anatomical-functional integrity of the intestinal mucosa and for greater safety of administration.
6. Discussion
Comparing the analysis of the real-world experience of managing the child with EB and the review of the literature, there is a huge correspondence between what the evidence identifies as the best care management and how healthcare professionals have actually acted.
The figure of the nurse in this rare condition, complex in the daily management, has a pivotal role, being responsible not only for skin management, wound dressing, prevention of infections and pain assessment and management, but also for eventual medical devices, such as central venous or urinary catheter and nasogastric tube.
Unfortunately, these patients often need central venous catheter for the administration of analgesic drugs, antibiotics, and possibly also for total parenteral nutrition, when enteral feeding is no longer tolerated due to gastrointestinal lesions. The bladder catheter is sometimes necessary to ensure adequate urinary elimination in case of urethral stricture caused by the lesions in the urinary tract. The nasogastric tube is placed to administer enteral nutrition as long as the child clinical conditions allowed its use.
In the case presented, all the precautions for isolation from contacts envisaged by hospital protocol were implemented to prevent the onset of infections. Also in this case, the nurse, following the indications of the dermatologist specialist, treated the lesions by assessing their condition from time to time and using all the devices deemed most suitable, such as disinfectant, topical antibiotic for the treatment of infected wounds on suppurating lesions and spray dressing with hyaluronic acid and metallic silver to allow healing. Finally, non-adherent dressings, such as vaseline gauze, were used to prevents adhesion to wounds and to allows a non-traumatic dressing change.
Regarding pain assessment and management, the FLACC scale was used, which is considered the most effective for the neonatal population. This tool consists of 5 items, including facial expressions, legs, activity, crying, consolability. A score ranging from 0 to 2 can be assigned for each of these indices, and the therapy to be administered is decided on the basis of the total score. If the score is between 0 and 4, the pain will be reevaluated after 60 minutes. Finally, if the score is > 4, analgesic therapy will be administered and pain will be re-evaluated, after 30 minutes if the therapy is administered parenterally and after 60 minutes if the therapy is administered enterally [10-12].
For pain, in combination with drug therapy, non-drug treatment was used; the analgesics were administered according to the current WHO guidelines, that identify three levels of pain and indicate for each of them the correct drug to use [26]. In Italy, The Italian Ministry of Health has recently published the “Guidelines for implementation of the Painless Hospital”, simplifying the legislation for prescribing opioid analgesics [27]. In the presented case, morphine and clonidine in continuous, administration of morphine boluses initially enterally, then intravenously, before wound dressing and as needed, have been used.
Finally, in EB repente infections, malabsorption and higher caloric intake may further complicate the natural history of the disease and therefore must be deeply considered in the daily management.
7. Conclusions
This paper focuses on the management of a rare, but extremely serious disease, with great care complexity.
The figure of the nurse in this path assumes huge rilevance, as it is daily interested in the care of this special patients, that need continuous and dedicated assistance from skilled professionals.
EB is a systemic pathology that is difficult to approach; it must be faced day by day, with repetitive but life-saving procedures, which must be properly put into practice by qualified personnel.
It is important that the caregiver of a child with EB is able to correctly assess the type of lesion present on the skin, has a good knowledge of the aids to use, according to the different entity of the wound.
Furthermore, in all disorders that force the person affected to live with chronic pain, it is important to have good pain management, helping those affected to have the best possible quality of life, starting from the neonatal age throughout life.
Competing Interests
The authors declare that they have no competing interests.