1. Introduction
Autism Spectrum Disorder (ASD) refers to a group of neurodevelopmental disabilities developing in the first few years of life, causing significant social, communicative and behavioral difficulties. ASD is usually assessed from 18 months of age, though in some developed countries the average age of assessment is as late as 30 months of age. However, as a neurodevelopmental condition unfolding in time infants at high risk for autism may be assessed before the full manifestation of the disorder. The prodrome of autism is characterized by impairments in the emergence of neurodevelopmental precursors associated with the onset of autism. The assessment for the prodrome of autism may occur from 12 months of age and even earlier in severe instances. The first two years of life presents a therapeutic window of opportunity for early intervention for autism and its prodrome during the period when the human brain is still undergoing rapid change and development.
This study focused on identifying developmental variables associated with autism among a group of infants later diagnosed with autism. Analysis of data from this study elicited a number of core developmental variables associated with the later onset of autism. Results from this study indicate that developmental symptoms associated with the prodrome of autism may be assessed already within the first year of life.
There are as yet no clear biological markers for ASD. Nonetheless, behavioral and developmental markers associated with autism have been identified among infants within the first year of life [2-8]. Infants with ASD can be distinguished from typically developing infants already from 12 months based on a combination of lack of typical behaviors, and the presence of atypical behaviors [9]. In their review of the prodrome of autism, Yirmiya and Charman [10] have identified prodromal antecedents to autism in three domains, including: delays and impairments in social communication and social relating behaviors, as well as the earliest emerging signs of rigid and stereotypical behavior. Two decades ago when parents of children with autism were asked when they first noticed that something was irregular in their child's development, they expressed either two typical responses: first was, ‘our child developed normally until the age of one year and then the regression began,’ and second, mostly by mothers, ‘I felt from the very beginning that something was wrong with my baby, but everyone said that I was a hysterical mother…’The following pioneering study was established to elicit what occurred among infants at the age of one associated with the later manifestation of autism. This study was intended to evaluate whether early signs associated with the later development of autism could also be detected amongst infants in both of these reported categories.
2. Methods
This study conducted over a period of a decade at the Mifne Center, examined 110 infants who were diagnosed with autism between the ages of 2-3 years, using retrospective analysis of video-recordings of the first year of their lives. The videos were filmed by the children’s’ parents before any suspicion concerning defective development arose. Since all the toddlers in this group were diagnosed with autism between the ages 2-3; their first year of life was analyzed as a self-control group. Data was analyzed in terms of individual variables and combinations of variables. The variables investigated in the study were developmental characteristics associated with the autism spectrum, and were measured according to parameters of time and frequency of the phenomenon.
Parents arriving for an interview with children who had been diagnosed on the autism spectrum, were asked whether they had videos of their child from the first year of life. Videos were consequently obtained of 110 infants who were consistently monitored by their parents before any suspicion concerning their infant’s development arose. The resultant retrospective video study conducted over a decade examined video recordings during their first year of life of 110 infants from various countries, who were diagnosed with autism between the ages of 2.5-3 years. 84 (76.4%) boys and 26 (23.6%) girls between the ages of 3-15 months from Europe, the United States, Australia and Israel participated in this study.
In addition to the video analyses, infant developmental information was collated via parental questionnaires. Developmental questions included, for example: When did you first notice your infant’s development problem? Who noticed the problem? How did you react? An anamnesis of the infant’s eating and sleeping habits was also obtained.
There were 2 criteria for inclusion of children in this retrospective evaluation of home videos included: (a) The home videos sent by the parents covered a period from at least 3 months old until 15 months at the latest. (b) All the children who were filmed on the videotapes received a diagnosis of autism between the ages of 2.5 to 3 years. Parents who agreed to participate in the study signed a consent form when delivering the videotapes. The videos were encoded according to monthly periods of time from 3 months, 4 months etc.
Four researchers who were expert in child development and who did not know the children watched the videos in pairs of two.
Each pair of researchers observed the videos separately and were instructed to note for any variables that appeared to depart from typical infant developmental behavior. The DSM-IV [11] provided the baseline categories of behavioral variables associated with the autism spectrum. However, these categories needed to be supplemented since the criteria mentioned in the DSM-IV related to older children above the age of 3 years.
Investigated variables were listed according to the following categories: Developmental variables elicited during the process of this study included: Lack of eye contact, lack of reaction to the presence/ voice of parents; excessive passivity; motor developmental delay; excessive activity; refusal to eat; aversion to touch; and accelerated growth of head circumference.
The variables in these categories were further evaluated in terms of age-appropriate behavior and context. It is important to stress that a number of the variables associated with early symptoms of autism detailed in this study were developed through the research process, and did not exist in their full clinical descriptions prior to the study’s completion, e.g. passivity, eating disorders, head circumference.
All the above variables were measured according to the following parameters:
0 = The sign does not exist (during observations)
1 = Doubting whether the sign exists (the observer is unsure)
2 = The sign rarely appears (not in every meeting)
3 = The sign does not appear consistently (once or twice in a meeting)
4 = The sign appears quite often (a few times during a meeting)
5 = The sign is very obvious (appears consistently)
In accordance with the aim of this study, the statistical analysis focused on identifying the most striking and common symptoms associated with the development of autism and the paired correlates between them. The procedure occurred accordingly in two phases. In the first phase, a baseline evaluation was conducted of the frequency of the identified indicative variables that were reported to occur amongst the infants. This statistical procedure was intended to identify those indicative variables occurring frequently among the infants, as well as those variables that occurred infrequently amongst a small portion of the investigated infants.
3. Results of Study
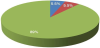
6 infants (5.5%) were diagnosed with pathologies; 6 infants (5.5%) showed no signs of developmental disorder during their first year of life. 98 infants (89%) showed early signs associated with the autism disorder between 5 to 15 months old (Figure 1).
3.1. Description of the elicited variables (Figure 2)
3.1.1 Lack of eye contact
The infant’s eye contact with the parents or other caregivers was examined. Measures of their tendency to follow the movement of persons with their eyes included: in which direction did the infant look? For how long? When did the infant lower their gaze? Did the infant ignore the person? Eye contact during closeness, eye contact during feeding, eye contact during play, or averting gaze [12,3].
From the very beginning, human infants are sensitive to social stimuli, especially faces [13-15], and from 3 to 5 months their gaze and affective behavior reflect increased sensitivity to their partner’s slight deviations in gaze and facial expressions [16,17]. Indicators of brain functioning may be sensitive predictors, and atypical eye contact is characteristic of the syndrome [18]. A prospective longitudinal study of infants with and without familial risk for autism was carried out with infants at 6-10 months, who were recorded in response to viewing faces with eye gaze directed toward versus away from the infant. This analysis showed that characteristics of ERP components evoked in response dynamic eye gaze shifts during infancy were associated with autism diagnosed at 36 months [18]. Responses to eye gaze may help characterize developmental processes that lead to later emerging autism.
3.1.2 Lack of reaction to the presence/voice of parents - (calling of their infant’s name)
There is no turning of the head, there is no deflection of the infant’s gaze, no smile or babbling, there is no attempt to divert attention. There is no reaction to the parents’ departure. What was evident from the video analysis, was that the infants who did not react to voices also did not react to presence, and vice-versa. There were no infants amongst those examined who reacted to presence but did not react to voices, something that characterizes babies with hearing loss. Nevertheless, singing almost always constituted a precipitating factor.
The majority of the research concerning the development of social and affective behavior as concentrated on cortical-related activity, mostly the amygdale-limbic system and the medial prefrontal and frontoparietal systems [19]. Despite this compelling framework, no direct evidence has yet been presented for the involvement of brainstem projections in the social engagement of humans, particularly of human infants. Early brainstem dysfunction detected during its major maturational spurt in the late prenatal period will directly affect the modulation of gaze as a function of arousal to social stimuli, thereby compromising social engagement [20].
3.1.3 Excessive-passivity
This variable was defined in cases of: lack of crying, lack of movement, lack of curiosity, lack of interest in what is happening in the surroundings, do not track people with their gaze, lack of effort to attain an object, there is no initiative, nor complain, “an easy baby” [3].
3.1.4 Motor development delay
Did the infant roll over; crawl; stand; walk at the appropriate development times; was the infant hypotonic? Although this variable was not significant in relating to autism, motor disturbances appear to play an intrinsic part in the phenomenon of autism, and can be used to diagnose the risk of autism in the first few months of life [21].
The association between impaired motor development and autism appears to have a genetic basis. For example, in his research on movement in first days old mice, Guy Horev [22] claims that in typical motor development the mice spread out four limbs to form a stable support base, while mice with inappropriate development were observed as having a different motor phenotype, whereby they contracted their arms and lay on an unstable base of support.
The Simons Simplex Collection [23] showed that damaging de novo mutations are significantly correlated with measures of impaired motor skills.
3.1.5 Excessive-activity
Consistent restless movement, consistent crying, obsessive occupation with an object, measured according to parameters of time and frequency of the phenomenon [24]. Analytic observation of all the components of the infants’ development pointed to lack of regulation, which were expressed in many cases by excessive-activity.
Certain brainstem functions, essentially the ABR function, first emerge around 30-33 weeks’ gestation, a period at which many premature births occur. This period is a critical one for major developmental changes in the equilibrium and the auditory pathways in the brainstem, including myelination [25]. Disruptions in myelination may disrupt white matter maturation and the integrity of neural connectivity and synchronization of neural oscillations. These in turn may bear implications for the establishment of emotional and attentional functions. Brainstem injury has been found to disrupt physiological regulation and homeostasis. These disruptions impact the autonomic nervous system, [26]. They also affect circadian arousal regulation, as well as visceral homeostasis modulation of internal states, such as hunger and thirst [27]. All of these systems are interrelated and moderate emotional and attentional regulation in infants during the neonatal phase [28]. Prospective follow-up showed poorer attentional responses in these infants that were hyper-responsive to increased endogenous arousal at 4 months [29].
3.1.6 Refusal to eat
Abnormalities in eating habits are listed as associated features of the autism disorder [30]. Most of the children who have been diagnosed on the autism spectrum display characteristics of eating disorders. The DSM-5 [1] mentions eating difficulties as one of the criteria associated with autism. Nutrition difficulties are common in children with autism from the early stages of life.
‘How we eat and how we learn to eat are important, so is what we eat’ [31]. Food refusal is defined as the consumption of fewer than the number of calories necessary or the rejection of food [32,33]. Food over-selectivity is defined as choosing only a limited number of food stuffs to be consumed or consuming an inadequate variety of food stuffs [34]. Most of the eating problems in children with autism can be included in the category of behavioral and sensory disturbances [35,36].
The development of eating skills is influenced by several integrative factors: the infant’s anatomical development and growth, the infant’s medical state, the infant’s emotional and social development, and type of environment [37]. Disorders of eating behavior in infants are represented by a range of patterns that include: An abnormal sucking reflex; rejection of breast feeding; refusal to eat; difficulty in swallowing (sometimes as a result of feeding via a tube); persistent and excessive spitting up (reflux has been cancelled out); lack of adjustment to solid food; abnormal chewing motion; fixation on minimal variety of foods; lack of curiosity regarding food; and aversion to various smells and textures. Many children are afraid of change in the food that accumulates in the mouth, afraid of chewing that causes the food to disappear. A sense that cannot be contained and may cause feelings of guilt and anxiety [35]. The fear of loss of the food is also connected with problems in sensory functions involved in the planning of the movement [38,39].
3.1.7 Aversion to touch
Physical touch relates to the amount of mass and physical proximity that the infant allows [40]. The definition of recoil relates to contraction or arching back, an expression of dissatisfaction when the infant is held, cuddled or kissed. The difficulty in allowing contact usually stems from a tactile overload that makes the infant feel uncomfortable or even pain. Difficulties in sensory processing characterize autism. Although for decades the relevant literature reported a lack of responsiveness as a sensory deficiency, in the last two decades it has become clear that this is actually over-sensitivity in the tactile, visual, audial and oral systems. Since this involves a lack of integration between the systems, there is no sensory modulation [41]. A lack of integration among the systems, causes difficulties in sensory modulation. Lack of sensory modulation may create confusion and anxiety, as well as tactile excessive sensitivity [42].
3.1.8 Accelerated growth of head circumference
Head circumference was examined according to the Mother and Baby Station records. According to the studies of Eric Courchesne [43,6], brain overgrowth in males with autism involved an abnormal excess number of neurons in the PFC, which is also responsible for emotional and behavioral processing.“Normal brain development is not a monologue but a dialogue, in which the brain generates neural circuits and the child's experiences determines which ones survive” [2]. Although prefrontal abnormality has been theorized to underlie some autistic symptoms, the cellular defects that cause abnormal overgrowth remain unknown.
3.1.9 Analysis of paired variables
In the second phase, a cross-sectional study was conducted comparing each identified sign against all the others, in order to identify “pairs” of signs, i.e., pairs of variables whose common occurrence was clearly apparent.
The study findings showed the possibility that early screening of risk for autism may be based on the ability to identify some combinations of indicative symptoms, which helps increase reliability. Moreover, as mentioned earlier, each of the identified variables were compared with each other in order to identify pairs of variables whose common occurrence was clearly apparent (Table 1).
The columns show the variables for couples, each of which represents a percentage of the variable both in the first and also in the second column. In older ages the weights of these variables may alter.
According to the findings, lack of eye contact was found to have a correlation of 35.5% among the studied children in combination with excessive passivity, while lack of reaction to parental presence showed a correlation of 37.3% in combination of lack of reaction to presence or voice with lack of eye contact and 21.8% showed a combination of excessive activity with lack of eye contact. In contrast, there are fewer common clusters such as aversion to touch and excessive passivity that only appeared in 2.7% of the investigated infants. These findings indicate that the clinical expression of a case of autism appears as a variety of clustered symptoms so that the possibility of accurate diagnosis depends more on the ability to identify these different clusters.
Key variables that were identified included: passivity, eye contact and motor development. It is likely that these paired variables constitute important factors in the assessment of the infants included in this study, and may be generally applicable to other infants at high risk for autism.
4. Discussion
This study provides a retrospective video analysis of infants during their first year of life before any neurodevelopmental abnormalities were detected, and who between the ages of 2-3 years were subsequently diagnosed to be on the autism spectrum. It is a pioneering study in terms of analysing signs of autism in the first year of life, and is singular in analysing infants who received a diagnosis of autism rather than focusing on siblings of children with autism - as characterizes most of the research literature around early assessment.
The analysis of its findings indicates that it was possible to identify early symptoms related to the autistic spectrum disorder already during the first year of an infant’s life. The 8 identified neurodevelopmental variables associated with the prodrome of autism included: excessive passivity, excessive activity, lack of eye contact, lack of reaction, refusal to eat, aversion to touch, motor development delay and rapid head circumference. The findings highlighted the prevalence of these signs for the group of 110 infants during their first 5-15 months of life. However, evaluating the clinical significance of these signs solely in terms of their linear relation with the prodrome of autism is of relatively limited significance. Therefore, the study also examined the possibility that early diagnosis of autism depends on the ability to identify various combinations of these indicative symptoms. The comparative findings indicated that the clinical expression of a case of autism appears as a variety of clustered symptoms, rather than a fixed set of diagnostic criteria, so that the possibility of accurate diagnosis depends more on the ability to identify these different clusters. The significant coexistence of these key variables can constitute valuable factors in the very early diagnosis of infants with autism.
As long as there are no proven biological markers for autism and its prodrome, the early diagnosis of ASD is necessarily an imprecise science. Standard criteria, such as the previous DSM-IV and DSM-5 as well as expert clinical judgment have rarely been applied to children under the age of 2 years [44]. Additionally, the stability of ASD diagnoses under the age of 2 is not well established and different studies have produced variable results [45-49]. Nonetheless, there is increasing evidence regarding the possibility and necessity of early diagnosis and intervention. Taking cognizance of the growing data pertaining to the early diagnosis of autism, the DSM-5 states that, ‘symptoms are typically recognized during the second year of life (12-24 months of age) but may be seen earlier than 12 months if developmental delays are severe or noted later than 24 months if symptoms are subtle’ (2013, p. 55). From an epistemological perspective, it is important to recognize that assessment for the prodrome of autism is not simply assessing risk for the later development of autism, but assessing a real neurodevelopmental condition that is unfolding in time. It is this temporal dimension that undergirds the therapeutic imperative for early assessment and intervention. From a clinical perspective, systematic screening of infants for autism at well-baby check-ups (12- 24 months) is an essential goal supported by the American Academy of Pediatrics. Even with screening at 12 and 24 months, children in many countries unfortunately do not begin treatment until well after their second or third birthday [7].
The eliciting of neurodevelopmental variables associated with the prodrome of autism in this study provided the basis for the Mifne Center developing an early assessment screening scale for autism [4]. This infant directed screening scale is called ESPASSI© (Applying the Early Signs of Pre-Autism Screening Scale in Infants) and contains the eight variables described: Lack of eye contact, lack of reaction to the parental presence; excessive passivity; motor developmental delay; excessive activity; refusal to eat; aversion to touch; and accelerated growth of head circumference. ESPASSI© is generally used from between 5 to 15 months.
Other early autism screening instruments besides the ESPASSI© have been proposed. For example, the Autism Observation Scale for Infants AOSI is a 19-item direct observational measure designed to detect and monitor signs of autism in infants aged 6-18 months. The behaviors examined include visual tracking and attentional disengagement, coordination of eye gaze and action, imitation, early social-affective and communicative behaviors, behavioral reactivity, and various sensory-motor behaviors [50,51]. The Communication and Symbolic Behavior Scales Developmental Profile has been designed for screening and evaluation of communication and symbolic abilities of children between 12 and 24 months of age [52,53]. The Checklist for Autism in Toddlers (CHAT) was developed to screen children for autism at age 18 months. It draws on evidence that impairments in social orienting behaviors (specifically joint attention behaviors) and pretend play may differentiate preschool children with ASD from children with general developmental delay. The CHAT is based on three key psychological predictors, namely, a lack of pretend play, a lack of proto-declarative pointing, and a lack of gaze monitoring. The presence of two or more of these predictors at 18 months is predictive of a later diagnosis of autism [54,55]. The M-CHAT - Modified Checklist for Autism in Toddlers [56] is built on the CHAT, and consists of 23 yes/no items reported by parents.
These various screening instruments share similarities in the main components they include to evaluate the manifestation of the prodrome of autism. Their respective variables all are intended to evaluate behavioral variables for emotional responsiveness, sensory behaviors and social engagement. The major differences between them are constituted depending on the age at which they focus their attention. Even at the age of 18 months and beyond, none of the screening instruments are conclusive. As Yirmiya and Charman have noted, ‘in none of the studies that have included systematic follow-up of the screened populations to identify all cases at outcome have the signs been universal. Scientifically such signs are promising but neither universal nor necessarily specific markers of a later emerging ASD presentation’ (2010, p. 439). Analysis of the results of the screening instruments indicates that pre-autism is expressed as a ‘variety of clustered symptoms,’ rather than a fixed set of diagnostic criteria [5]. It is important to emphasize, that a positive assessment does not put a label of autism on a very young infant. As utilized throughout this paper, the term the “prodrome of autism” refers to infants at high risk for autism and defines a temporally unfolding neuro-developmental condition, which by definition is not yet autism, but is characterized by impairments in the emergence of behaviors associated with autism.
5. Conclusion
The pioneering study described in this paper demonstrates the possibility of eliciting symptoms associated with very early screening within the first year of life, in some cases from as early as 5 or 6 months of age where there is cause for concern. It is already well accepted that early signs of autism may be detected in the second year of life. The most recent edition of the DSM-5 recognizes the possibility of early detection of autism among infants from 12-24 months and sometimes earlier if the symptoms are severe. The development of effective early screening assessments for infants with autism is, however, a necessary, but insufficient step in the treatment of very young infants with autism. The dynamic development of the brain during the first two years of a baby’s life is critical with regard to the baby’s ability to assimilate stimuli from the environment lay down new neural pathways. The exploitation of this therapeutic window of opportunity through the use of suitable stimulation may influence the development of neural connections and thus contribute to the possibility of minimizing the severity of the phenotypic presentation of autism. Together with early assessment a therapeutic imperative exists, therefore, to develop and research effective interventions for infants in the first 2 years of life diagnosed with the prodrome of autism.
However, there exist at present only a few published studies analysing the evidence-based efficacy of very early treatment interventions for infants with ASD. A comparative study described in the following chapter aimed to provide information regarding the effectiveness of early intervention dependent on the child’s age.
Competing Interests
The authors declare that they have no competing interests.