1. Introduction
Multiple sclerosis (MS) is a chronic demyelinating condition characterized by active and dormant foci of central nervous system demyelination with neurological manifestations disseminated across time and space [1]. It is the most common demyelinating disease in humans with an estimated worldwide prevalence of 30.1 cases per 100,000 with the highest prevalence found in North America [2]. Neuropsychiatric symptoms have been found to be associated with MS and can present both during and between MS exacerbations [3-5]. Onset of MS before the age of 16 is unusual, constituting an estimated 2.2% of cases [6]. In the pediatric population, it has been estimated that up to 48% of patients with a demyelinating disorder demonstrate such psychiatric symptoms as affective disorders and psychoses [7]. We present the case of 14-year-old patient incidentally diagnosed with multiple sclerosis following a near-successful suicide attempt that was preceded by a two-year history of suicide attempts, suicidality, and neuropsychiatric symptoms.
2. Case
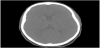
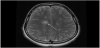
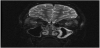
A 14-year-old female was found by her mother hanged by a belt at home. The patient was cut down by her mother and found to be unresponsive. Cardiopulmonary resuscitation was initiated at the scene and return of spontaneous circulation was achieved upon arrival of ground EMS. The patient was subsequently intubated and airlifted to our facility. On arrival, ligature marks to the neck and flexor posturing were noted. Pupils were 4-5 mm in diameter and sluggishly reactive, rectal tone was normal, and GCS score was 3T. Superficial, well-healed linear scars were noted to the left wrist. Chest x-ray and CTA of the head and neck were obtained on hospital day 1 to evaluate for edematous or ischemic changes and revealed hypodensities in the caudate and lentiform nuclei and the deep white matter superior to the lateral ventricles (Figure 1). There was no evidence of injury to the vasculature or axial skeleton and no overt signs of anoxic brain injury. A correlating MRI of the brain and cervical spine was obtained on hospital day 2. FLAIR images demonstrated multiple elongated callosomarginal hyperintensities with involvement of the basal ganglia and brainstem. Three of these lesions showed surrounding vasogenic edema and a ring enhancement suggestive of active demyelinating plaques (Figure 2).
Collateral interview was performed with the patient’s parents and a concerning trend of progressive psychiatric and neurologic dysfunction was revealed. The patient lived in a stable two-parent home, had close relationships with friends and family, and was sociable and outgoing at baseline. Two years prior to presentation, at 12 years of age, the patient first expressed suicidal thoughts and clandestinely obtained and consumed six azithromycin tablets, reportedly stating at the time that she “didn’t expect to wake up.” At 13 years of age, the patient began to experience transient loss of vision, occurring up to three to four times per day. The patient’s teacher began reporting episodes described as behavioral arrest with blank staring spells lasting a few seconds at a time and associated with amnesia of the event. These episodes were not associated with abnormal movements, lip smacking, tongue biting, or sphincter dysfunction (We would eventually learn from the patient that she had experienced similar episodes for two years, with each episode followed by head pain, altered mentation, and amnesia of the event). Additionally, over the first two months of 2020, the patient complained of bilateral leg pain relieved with activity and weakness after hot showers. During this time, the patient began articulating, with pressured speech, her intention to commit self-harm with daily frequency. She began to demonstrate lapses in concentration and impaired word-finding. The patient’s psychiatric and neurological symptoms reportedly waned between the months of March and April 2020, two to three months following the initial onset of episodic vision loss. However, in May 2020, the patient once again attempted suicide by ingestion with naproxen. She was admitted to a behavioral health center for treatment and was diagnosed with major depressive disorder, anxiety disorder, and absence seizures. She was discharged on 150 milligrams oxcarbazepine and 50 milligrams sertraline daily. Thereafter, the patient’s psychiatric condition further deteriorated. She became physically aggressive with her sister and emotionally labile, misinterpreted dreams as reality, described demonic hallucinations, and demonstrated hyper-religious confabulation. In late July 2020, she called 911 on her father, falsely claiming that he had strangled her with a telephone cord and that she intended to end her life. There were no further acute events until October 2020, when the patient presented to our facility following attempted suicide by asphyxiation. In the moments preceding the attempt, she made statements to her mother to allay suspicion and to her father to ensure that he was preoccupied. The sophisticated design of the asphyxiation apparatus further suggests that the attempt was planned.
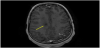
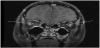
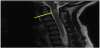
Following extubation on hospital day 3, the patient was noted to have slowed speech, confusion, emotional lability, and abnormal thought content. MRI with contrast of the brain and orbits at that time revealed multiple ovoid T2 hyperintensities with ring enhancement, as well as increased STIR signal intensity and contrast enhancement in the bilateral optic nerves (Figures 3-5). By hospital day 5, the patient was still demonstrating an expansive affect, alternately laughing and crying and making statements inappropriate to the situation. Her thought content was rife with religiosity, at one point claiming to see and talk to God. She described people in the room who were not present. She was started on a five-day course of methylprednisolone for radiographic evidence of cervical edema (Figure 6). An EEG performed at this time was unremarkable for ictal or interictal activity. However, during the evening of hospital day 5, the patient experienced two generalized tonic-clonic seizures lasting less than one minute each. She was loaded with 40 milligrams per kilogram levetiracetam followed by 20 milligrams per kilogram maintenance doses twice daily. Three episodes of generalized shaking were observed on hospital day 6, each event lasting less than 15 seconds and prompting an increase in levetiracetam maintenance dosing to 40 milligrams per kilogram twice daily by hospital day 7. By hospital day 8, the patient still endorsed amnesia of the events leading to her hospitalization but denied ideation of self-harm. Serum ammonia assay and COVID-19 screen were normal. Lumbar puncture was significant for elevated oligoclonal bands and increased kappa light chains. CSF analysis for angiotensin-converting enzyme (ACE), Venereal Disease Research Laboratory (VDRL), Lyme serology, cryptococcal antigen, autoimmune encephalitis, and Neuromyelitis optica/ Aquaporin 4 immunoglobulin G (NMO-AQP4-IgG) were negative. CSF and urine cultures were negative and CSF cytology was negative for malignant cells with and was notable for an increased lymphoid population and plasma cells.
Incremental progress in the patient’s cognition, psychosis, and physical functioning was noted between hospital days 10 and 13. By hospital day 14, the patient was able to independently perform schoolwork. The patient’s planned discharge to an inpatient rehabilitation facility was delayed by insurance authorization and bed availability. By hospital day 17, the patient had improved sufficiently to be cleared for discharge home by the hospital medicine team, psychiatry team, and physical therapy team. Arrangements were made for follow-up with child psychiatry, behavioral therapy, and neurology. She returned to our facility one week later for initiation of therapy with fingolimod and cardiac monitoring, with no adverse neuropsychiatric events reported in the interval.
3. Discussion
Neuropsychiatric findings have been found to be present in as many as 95% of MS patients, with major depression being the most widely observed psychiatric manifestation [3]. The prevalence of major depressive disorder in MS patients has been found to be up to 5 times higher than that found in the general population [8,9]. Though our patient was initially diagnosed with major depression and anxiety, her hypomanic behavior, depressive episodes, increased aggression, propensity to self-harm, and psychotic features, all deteriorating concurrently with neurological symptoms, suggest an alternate psychiatric diagnosis. Bipolar disorder with psychotic features, schizophrenia, and schizoaffective disorder led our differential diagnosis.
Like major depression, bipolar disorder is a well-characterized psychiatric condition associated with MS, occurring as much as two times as frequently in MS patients as in the general population with a prevalence of 5.8-10% in patients with MS [8,10]. It appears to be unusual that the neuromotor symptoms of MS would present in a patient prior to the onset of bipolar symptoms. A review of 26 cases performed by the University of Ferrara in 2015 showed that bipolar symptoms preceded the onset of MS symptoms by 5 years, but in only one case were bipolar symptoms identified in a patient less than 20 years of age [11]. Bipolar disease in the pediatric MS population has not been extensively described and understanding of how to manage this condition in such patients is correspondingly scarce.
Schizophrenia has also been extensively linked with multiple sclerosis and associations between the two conditions with regard to gender, race, geography, season of onset, season of birth, and genetics have been postulated to exist [12,13]. A comparable pathogenesis of the two conditions has been suggested, citing an autoimmune model of acquisition, but conclusive evidence supporting this relationship is lacking [13]. It is estimated that 2-3% of patients with multiple sclerosis present with psychotic symptoms [14]. A 2017 literature review found that schizophrenia was diagnosed in 13.2% of patients with MS who presented with a psychotic disorder [15]. Schizoaffective disorder was identified in 8.8% of these cases, whereas psychotic depression (14.3%), bipolar I with psychotic features (10.9%), and bipolar II with psychotic features (12.1%) were also identified [15]. Of these patients, 24.1% demonstrated psychotic symptoms prior to diagnosis with MS, as was the case with our patient [15].
Prior to her arrival at our facility following an unsuccessful suicide attempt, the patient had been prescribed oxcarbazepine for management of what was believed to be absence seizures in the context of a psychiatric disorder. A 10-keto analogue of carbamazepine, oxcarbazepine is generally prescribed for the management of epilepsy but has also been used for the management of neuropsychiatric conditions including affective and mood disorders [16,17]. The efficacy of oxcarbazepine for the management of bipolar disorder in pediatric patients has not been extensively evaluated, though one 2006 multicenter trial found that oxcarbazepine was not significantly more effective than a placebo in the treatment of bipolar I disorder in children aged 7-18 [17,18]. Oxcarbazepine is not generally used for the treatment of schizophrenia, though there is limited evidence that it may have a benefit as adjunctive therapy in this condition [19]. The side-effect profile of oxcarbazepine has been more widely investigated and came under scrutiny following a 2008 study from the United States Food and Drug Administration linking antiepileptic drugs (AEDs) with an increased risk of suicidality [20]. However, a subgroup analysis within this study of patients using AEDs for psychiatric conditions showed no significant correlation with suicidality, nor did the use of oxcarbazepine when prescribed for any indicated condition [20,21]. A more focused 2009 study examining the relationship between suicide attempts and AED use in patients with bipolar disorder revealed no significant relationship between AED use and suicide attempts even when adjusted for concomitant therapy with antidepressants. Moreover, this study demonstrated that oxcarbazepine showed the greatest difference in pretreatment versus post-treatment suicide attempts [21]. We believe that it is, therefore, all the more unusual that our patient’s psychiatric condition continued to deteriorate following initiation of therapy with oxcarbazepine. Though the use of carbamazepine in MS has been associated with a greater risk of adverse neurological effects compared to other medications [22], the neuropsychiatric effects of oxcarbazepine in pediatric MS patients with an associated neuropsychiatric disorder has not been evaluated.
4. Conclusion
The patient’s persistent suicidality and multiple suicide attempts in the context of progressive psychiatric deterioration and neurologic decline prior to diagnosis represent a presenting manifestation of her underlying multiple sclerosis. We must also consider that the etiology of the patient’s pre-admission psychiatric symptoms was likely multifactorial. The social isolation secondary to the COVID-19 pandemic, the sociological pressures of beginning secondary school, and the emotional burden and physiological influence of an undiagnosed neurological condition cannot be understated. The uniqueness of this disease presentation within an adolescent should likewise be taken in context with the unique experiences of adolescence. We hope that this case presentation serves to further the understanding of the causes of suicidality in adolescents and prompts diagnosticians to consider underlying neurologic abnormalities in their evaluation of such patients.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Brian P. Logarbo: Composed and conceptualized manuscript,
performed literature review, assembled, interpreted, and analyzed
data, resident on primary hospital medicine team.
Pat F. Bass III: Edited manuscript, revised manuscript, interpreted and
analyzed data, provided academic counsel.
Adijat Olanrewaju: Interpreted and analyzed data, provided academic
counsel, attending physician on primary hospital medicine team.
Sheila J. Asghar: Conceptualized manuscript,edited manuscript,
revised manuscript,interpreted and analyzed data, provided academic
counsel, attending physician on pediatric neurology team.