1. Introduction
Pre-eclampsia, defined as the onset of hypertension and the presence of protein in the urine at >20 weeks of gestation in a previously normotensive woman, is a pregnancy complication that is still one of the leading causes of death and disability of both mother and babies. Pre-eclampsia occurs in 5-8% of pregnancies in developed countries.
Risk factors for pre-eclampsia that have been identified in previous studies include: both young and old maternal age, high BMI, prior pregnancy with pre-eclampsia, excessive weight gain during pregnancy, nulliparity, chronic hypertension, low socioeconomic status, prolonged birth interval, race and ethnicity, genetic predisposition, environmental and even seasonal influences. Ironically, although smoking during pregnancy causes various adverse pregnancy outcomes, when it comes to pre-eclampsia and hypertensive disorders in pregnancy, many studies have shown that it is associated with reduced risk.
The role of immune mechanisms contributing to the development of a normal pregnancy is widely discussed. Their involvement in the pathogenesis of pregnancy complications, such as preeclampsia, was also noted. The analysis of the scientific literature reveals conclusion that many aspects of the pathogenesis of preeclampsia are related with systemic inflammatory response syndrome with the development of a destructive inflammatory process, immune disorders, and the imbalance of cytokine regulation of gestation processes.Studies showed that in pregnancy complicated by preeclampsia, cytokine levels essentially change compared with the respective levels in physiological pregnancy. Thus, even a moderate form of preeclampsia shows directional change, i.e., elevated levels of pro- and antiinflammatory cytokines, with the exception of IL-10, wherein a downward trend in severe preeclampsia is recorded.
We carried out this study to estimate the risk of developing different forms of pre-eclampsia for each previously known demographic and clinical risk factor as to evaluate the relationship between the formation of anti-inflammatory IL10cytokine and several other biochemical indicators of moderate and severe preeclampsia.This will provide an evidence base from which healthcare professionals can assess each pregnant woman's risk of pre-eclampsia at her first antenatal visit and arrange her antenatal care according to need.
2. Prediction for Severe / Non-severE Pre-eclampsia
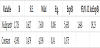
Uni-variant Logistic Regression Analysis for determination of the predictive role of certain socio - demographic, clinical and biochemical parameters for severe pre-eclampsia.
2.1 Maternal age
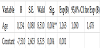
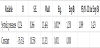
Maternal age of patients with non-severe and severe pre-eclampsia was analyzed into two categories: older than 35 and younger than 35 years. The results showed that 16% of the patients with nonsevere form of pre-eclampsia and 52% of the patients with severe form of preeclampsia were older than 35 years. The statistical analysis confirmed that pregnant women older than 35 years, highly significantly, have severe form of preeclampsia (p=0,007).
The age of the respondents analyzed as continuous variable has confirmed itself as highly significant predictor for severe form of preeclampsia (p=0,004). Advancing the age for another year increases the probability for getting severe form of pre-eclampsia during the pregnancy for 26,3% (95,0% CI 1.08 - 1,478) (Table 1).
Dependent variable - severe preeclampsia/ non-severe preeclampsia **p<0,01.
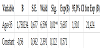
The age analysis as categorical variable in two age groups (older and younger than 35 years), have shown that pregnant women older than 35 years are in 5,687 times (95,0% CI 1,510 - 21,424) bigger risk from the pregnant women aged 35 and younger to develop severe form of preeclampsia (Table 2).
2.2 Gestation
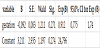
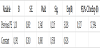
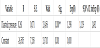
The probability for getting severe form of preeclampsia insignificantly decreases with the increase of the gestation length of the pregnant women (p=0,271). If the pregnancy continues for one more gestational week, the chance for getting severe form of preeclampsia decreases by 8,8% (95,0% CI 0,775 - 1,74) (Table 3).
2.3 BMI
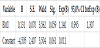
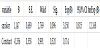
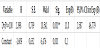
Although studies from developed countries show that high prepregnancy body mass index is associated with increased risk of preeclampsia, for level of significance of p=0,05, the results of this survey have confirmed that the value of the BMI is insignificant factor for severe pre-eclampsia (p=0,059) (Table 4).
2.4 Nulliparity
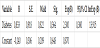
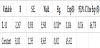
The nulliparity represents highly significant risk factor for severe form of pre-eclampsia (p=0,006). Pregnant women without history for previous delivery are in 5,63 times (95,0% CI 1,648-19,232) higher risk than the pregnant women who previously gave birth for getting a severe form of preeclampsia (Table 5).
2.5 Number of pregnancies
The number of pregnancies is insignificant risk factor for severe form of pre-eclampsia (p=0,882). Pregnant women with two pregnancies have 0,857 times (95,0% CI 0,111-6,617) insignificantly smaller chance than the ones with one pregnancy to develop severe form of pre-eclampsia (Table 6).
2.6 Previous preeclampsia
The results of our survey did not show that previous pre-eclampsia significantly increases the chance for getting severe form of preeclampsia (p=0,215). Pregnant women with history for previous preeclampsia have 3,028 times insignificantly larger probability from those with negative history for previous pre-eclampsia to develop severe form of preeclampsia (Table 7).
2.7 Smoking status
Smoking cigarettes insignificantly increases the risk for severe preeclampsia (p=0,096). Pregnant women that are smokers are in 3,15 times insignificantly higher risk than the pregnant women that are non-smokers for getting severe pre-eclampsia (Table 8).
2.8 Diabetes mellitus
Diabetes type 1, type 2 or gestation diabetes are insignificantly associated with severe form of pre-eclampsia in the pregnancy (p=0,364). The chances for getting a severe pre-eclampsia increases 2,3 times insignificantly for the pregnant women with diabetes mellitus compared with the pregnant women without diabetes (Table 9).
2.9 Systolic blood pressure
Systolic blood pressure form 160mmHg and higher is measured in 20% of the patients in the group with non-severe form of eclampsia, and in 92% of the patients from the group with severe form. The difference in the distribution of respondents with values of systolic blood pressure higher and lower than 160mmHg is statistically highly significant (p= 0,01) (Figure 2).
Pearson Chi-square: 26,29 df=1, p=0,000** p<0,01.
Systolic blood pressure analyzed as continuous variable has confirmed itself as highly significant predictor for severe preeclampsia in pregnancy (p=0,001). The increasing of systolic blood pressure for 1mmHg (95,0% CI1,009-1,423) increases the probability for 25% for severe pre-eclampsia (Table 9).
Pregnant women who have systolic blood pressure 160mmHg and higher have 46 times (95,0% CI8,027-1,423) significantly higher chance than pregnant women with systolic blood pressure lower than 160mmHg to develop severe form of pre-eclampsia (Table 10).
2.10 Diastolic blood pressure
Diastolic blood pressure of 100mmHg and higher more often had the respondents from the group with severe pre-eclampsia compared with the ones from the group with non-severe preeclampsia (88% vs. 44%) (Figure 3).
Pearson Chi-square: 12,5, df=1, p=0,0004** p<0,01.
Diastolic blood pressure highly significant can predict phenomenon of severe preeclampsia in pregnancy (p=0,000). The increasing of the diastolic blood pressure for 1mmHg increases the probability for severe pre-eclampsia for 29, 8% (95,0% CI1,129-1,492) (Table 11).
Dependent variable – severe eclampsia/ medium eclampsia **p<0,01.
Pregnant women who have diastolic blood pressure 100mmHg and higher have 11 times significantly higher chance than pregnant women with diastolic blood pressure lower than 100mHg to develop severe form of eclampsia (Table 12).
2.11 Level of IL-10 in serum
Study data demonstrated that in pregnant women with pregnancy complicated by preeclampsia, the serum concentration of antiinflammatory IL10 is confirmed as a significant predictor of the occurrence of severe preeclampsia. Increased serum concentrations of IL10 for one pg/mL reduces the likelihood of development of severe preeclampsia by 89.6% (95% CI 0.016-0.678). The sensitivity of this parameter as a predictor for severe pre-eclampsia is 96%, and the specificity is 80% (Table 13).
Dependent variable: severe preeclampsia.
Analysis of the relationships between serum maternal concentration of IL10 and serum concentration of enzyme LDH, creatinine, platelets, proteinuria, and uric acid was also made.
The obtained values of Pearson’s coefficients indicate negative correlations of IL10 with LDH and proteinuria, whereas the correlations of IL10 with creatinine, platelets, and uric acid were positive. However, significant correlations were confirmed only between IL10 and platelets as well as between IL10 and proteinuria. The correlation with the platelets count was positive which means that significantly higher concentration of IL10 was confirmed in patients with higher number of platelets in the blood, and vice versa. The correlation between IL10 and proteinuria was negative showing that the serum concentration of IL10 was significantly lower in patients with higher amount of proteins in the urine, and vice versa.
This study demonstrates differences in IL10 levels in women with preeclampsia compared to the levels in women with a normal pregnancy outcome. We found that in pregnant women with preeclampsia, the increased serum concentrations of IL10 predicted lower likelihood for the development of severe preeclampsia.
3. Discussion
One of the aim of our study was to define the demographic and socio-economic characteristics of pregnant women with the risk of pre-eclampsia in Macedonia. So as for this part of the study, the results showed that elevated systolic blood pressure of 160 mmHg or higher, diastolic blood pressure of 100 mmHg or higher, pregnancy at older age than 35 years as is nulliparity are associated with highly significant risk for developing severe form of pre-eclampsia. Other risk factors examinated in this survey such as duration of gestation, BMI, number of pregnencies, previous pregnancy with pre-eclampsia, diabetes and smoking status according to the results of this study, are risk factors that insignificantly increase the risk for severe form of pre-eclapmsia.
Next, knowing that the pregnancy is a condition that requires immunological tolerance and knowing that it is widely accepted that immune mechanisms are involved in pathogenesis of pregnancy complications such as pre-eclampsia, we examinated the correlation between IL10 serum concentrations (as a biochemical marker) and pre-eclamsia with its severity. Previous studies showed that in pregnancy complicated by preeclampsia, cytokine levels essentially change compared with the respective levels in physiological pregnancy. Thus, even a moderate form of preeclampsia shows directional change, i.e., elevated levels of pro- and anti-inflammatory cytokines, with the exception of IL-10, wherein a downward trend in severe preeclampsia is recorded.
4. Results
The regression analysis applied in this study showed that elevated systolic blood pressure of 160 mmHg or higher, diastolic blood pressure of 100 mmHg or higher, pregnancy at older age than 35 years as is nulliparity are associated with highly significant risk for developing severe form of pre-eclampsia. While other variables predicted higher likelihood for the development of severe preeclampsia, IL10 decreased such likelihood. IL10 was also found to be negatively correlated with proteinuria, and positively correlated with blood platelets. Significantly higher concentration of IL10 was confirmed in patients with higher number of platelets in the blood, and vice versa. On the other hand, the serum concentration of IL10 was significantly lower in patients with higher amount of proteins in the urine, and vice versa.
The actual study demonstrated platelets count and proteinuria as significant predictors of serum IL10 concentration - platelets count predicting higher serum concentration of IL10, while urine proteins predicting lower serum IL10.
5. Conclusion
Management of preeclampsia centers on early recognition and timely intervention to prevent serious morbidity and mortality. Despite recent advances in our understanding of the etiology of preeclampsia, there is still no clinically useful screening test. Since prevention of pre-eclampsia is not possible, the target should be to estimate the severity of the disease that will provide intensive supervision during the further course of pregnancy. That can be done by identifying risk factors that are associated with significant risk for developing severe form of pre-eclampsia and early recognition of the biochemical markers whose fluctuations also correlate with the risk for developing severe form of this disease.
Competing Interests
The authors declare that they have no competing interests.