1. Introduction
Neonatal sepsis is a type of neonatal infection and refers to the presence of a bacterial blood stream infection (BSI) (such as meningitis, pneumonia, pyelonephritis, or gastroenteritis) and it is the most important cause of morbidity and mortality in the neonatal period [1].
Preterm neonates are at risk of suffering from severe infections particularly in the first month of life due to their low immunity against infections. Immaturity of the immune system makes neonatal sepsis more common in preterm than full term and in Caesarean Section (CS) than spontaneous vaginal delivery (SVD) [2].
Early onset sepsis (EOS) is defined as the onset of sepsis during the first 3 days of life and is mostly caused by vertical transmission of bacteria from the mother to her infant during the intrapartum period [3]. Late onset sepsis (LOS) is defined as infection that occurs during the first week of life and is caused by the transmission of pathogens acquired postnatally and is often more insidious in onset [4].
Antimicrobial proteins and peptides (APPs) are cationic molecules that are released by neutrophils, monocytes, and macrophages. APPs are also produced within the skin and at mucosal surfaces by epithelial cells in the respiratory, gastrointestinal, and urinary tract. They are present in the body fluids, including saliva, tears, nasal secretion, gastric juice, sweat, semen, airway surface liquid, and breast milk.Human beta defensin 2(HBD2) is a clinically important APPs in early life [5]. HBD2 is a cysteine-rich cationic low molecular weight antimicrobial peptide first discovered in psoriatic lesional skin [6].
APPs were found to be lower in newborns than in adults. Although APPs could be important to protect newborns from infections during the first weeks of life, HBD2 are reduced in preterm infants due to the immaturity of the immune system [7].
2. Aim of the work
The aim of this work is to detect and evaluate HBD2 in cord blood on late onset sepsis in preterm, near-term and full term neonates.
3. Patients and Methods
3.1 Study design
This is a comparative follow up study.Forty neonates for each group were collected and studied at birth and then followed up(to exclude the presence of any bias) for one month for the development of signs of sepsis.
3.2 Population
This study was carried on 120 neonates from NICU of Minia University Hospital and from routine follow up neonates in Neonatal Outpatient Clinic, Minia University for the third group during the period of December 2015 to September 2016, who were divided into three groups:-
Group I: included 40 preterm neonates (GA <32 wks).
Group II: 40 late preterm neonates, (GA> 32 and< 37 wks).
Group III: 40 full term neonates (GA 37-40wks).
All neonates were subjected to the following: history taking; maternal history (history of previous pregnancies, maternal illness, antenatal care, drug intake, type of delivery (caesarean or vaginal). Neonatal history (sex, twins status, gestational age, birth weight), clinical examination; general condition, moro and suckling reflexes, presence of mottling, pathological jaundice, septic arthritis and laboratory investigations; C-reactive protein (CRP), Complete blood count, levels of cord blood HβD2.All neonates were followed clinically for one month for the development of signs of sepsis, with laboratory follow up of CBC, CRP, and serum HβD2 during a period of 30 days of age.
Exclusion criteria were maternal illness (pre-eclampsia, eclampsia, diabetes mellitus, epilepsy, infections), history of chorioamenitis, PROM, history of drug intake during pregnancy, congenital anomalies of neonates andobstructed labor.
3.3 Blood sampling and processing
2 ml blood samples were taken immediately after birth from the umbilical cord for detection of HβD2.
3.4 Blood sample of follow up
1.5 ml of venous blood was drawn from each neonate under complete aseptic conditions and divided as follow:
0.5 ml on EDTA tube for CBC (automated cell counter sysmix, NE), O.5 ml on an empty tube for CRP, the remaining 0.5 ml was centrifuged and separated serum was stored at -80 for detection of HβD2.
3.5 Human β-defensin 2 testing
The samples were diluted 1:20, and HBD2 concentrations were measured in sera, without protein extraction procedure, using an enzyme linked immunoassay (Phoenix Pharmaceuticals, Burlingame, according to the manufacturer’s instructions.
3.6 Statistical analysis
The collected data were coded, tabulated, and statistically analyzed using SPSS program (Statistical Package for Social Sciences) software version 20. Descriptive statistics done for numerical data were mean, standard deviation and range, while categorical data were represented as number and percentage.
Analyses were done for parametric quantitative variables using one way ANOVA test for comparison between three groups and post Hoc Tukey's correction between each two groups. Comparisons of quantitative variables between two groups were done using independent sample t test for parametric data, and Mann Whitney test for non-parametric quantitative data between the two groups.Chi square test was used to compare qualitative data.
Correlation between two quantitative variables was done by using Pearson's correlation coefficient, and correlation between quantitative and ordinal variables was done by using non-parametric Spearman's rho correlation coefficient.The level of significance was taken at (P value ≤0.05).
Correlation coefficient ranges from (0-1):- weak (r=0-0.24), fair (r=0.25-0.49), moderate (r=0.5-0.74), strong (r=0.75-1)8. ROC curve was done to determine the cutoff point, area under the curve (AUC), sensitivity, specificity, positive predicative value, negative predicative value, and accuracy of HβD2as predictive of late onset sepsis.
3.7 Ethics
Informed parental consent was obtained to be eligible for enrollment into the study. The study was done according to the rules of the Local Ethics Committee of Faculty of Medicine, Minia University, Egypt.
4. Results
This study was carried on 120 neonates from the NICU, divided into 3 groups:
Group I: included 40 preterm neonates (GA, <32 wks.), (28 (70%) males and 12 (30%) females). Thirty six developed LOS, whereas 4 did not as determined according to the Neonatal sepsis screening 7.
Group II: 40 late preterm neonates, (GA, > 32 and < 37 wks.), (24 (60%) males and 16 (40%) females). Eleven developed LOS while 29 did not develop sepsis.
Group III: 40 full term neonates (GA, 37-40wk), (32 (80%) males and 8 (20%) females), of whom 5 developed LOS and 35 did not develop sepsis.
Differences in weights, gestational age, maternal age and the mode of delivery are shown in table 1.
There were no statistical differences among the three studied groups with respect to hemoglobin(Hb), total leucocytic count (TLC) and platelets. As for CRP, it was positive in 22 neonates (55%) and in 61% of cases with sepsisin group I, 8 neonates (20%)and 72.7% of cases with sepsisin group II, and 4 neonates (10%) and 80% of cases with sepsis in group III (Table 2).
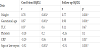
Cord blood levels of HβD2 showed the lowest level in group I, less than that of group II; the highest level was seen in group III (12.6±2.7, 17.4±2.1, 35.9±16.8 ng/L respectively) (P= 0.03, 0.001, 0.001 respectively) (Table 3).
Compared to their cord blood levels, the follow up levels of serumHβD2of both the late preterm (group II) and full-term (group III) groups showed significant increases; (22.4±4.1, 42.2±15.01ng/L respectively) (P= 0.001*) (Table 3) (Figure 1).
Cord blood HβD2 significantly correlated with weight, gestational age, and signs of late sepsis in group I, with gestational age, TLC, platelets, CRP, and signs of late sepsis in group II, and with weight, gestational age, CRP, and signs of late sepsis in group III (Table 4, 5, and 6).
ROC curve analysis showed that AUC (area under the curve) for cord blood HβD2 in prediction of sepsis among early preterm was (0.72±0.08) at cut off point ≤12ng/L with sensitivity and specificity of (72.7% and 55.6% respectively), and these values were significant (p=0.01*). (Table 7) (Figure 2).
ROC curve analysis showing that AUC for cord blood HβD2 in prediction of sepsis among late pre term was (0.95±0.02) at cut off ≤16.5ng/L with sensitivity and specificity of (82.8% and 100% respectively), and these values were significant (p=0.001). (Table 8 and figure 3).
ROC curve analysis showing that AUC for cord blood HβD2 in prediction of sepsis among full term was (0.94±0.04) at cut off ≤26ng/ Lwith sensitivity and specificity of (80% and 100% respectively), and these values were significant (p=0.001*) (Table 9 and figure 4).
5. Discussion
Defensins are cationic antimicrobial peptides contributing to the host defense against microbial invasion [10]. HBD-2 is effective against both Gram-negative and Gram-positive bacteria, such as E. coli and S. aureus, respectively [11].
Our study revealed that the frequency of LOS was significantly higher in preterm than both late pre-term and full-term; this is in agreement with the study of Utomo et al. [12], who stated that the incidence of LOS was higher in neonates with low birth weight than full-term neonates. Premature birth increases the susceptibility to serious infections, including blood stream infection (BSI), meningitis, and pneumonia [13].
Regarding CRP, it was positive (+ve) in 55% of preterm neonates, 20% in late preterm and 10% in fullterm. No significant differences regarding hemoglobin, TLC or platelet count among the three groups. This agrees with study of Mannan etal.,[14] who concluded that CRP is most sensitive method (93%) in culture proven sepsis and (79%) in suspected sepsis and its positive predictive value in suspected sepsis amounts to 88%, also, in their study, they stated that TLC didn't show any significant positive results but thrombocytopenia was present in 50% cases of culture positive sepsis.
In our study, levels of cord blood HβD2 were significantly decreased in preterm than both late preterm and full term.
In follow up levels of HβD2, there was also a significant decrease in their levels in preterm than both late preterm and full term.
There was a significant difference between cord blood HβD2 and follow up HβD2 in both late preterm and full term as the follow up HβD2 was increased as the immune system is mature. Yasuhiro et al., [15] and Wynn et al.,[16] stated that physiological low levels of the innate immune system (e.g., complement proteins including mannose-binding lectin) increases the susceptibility to infections in preterm neonates.
Studies of human fetal intestinal tissue supported that the APP levels are relatively diminished in early life: reduced mRNA expression levels of HD-5 and HD-6 have been detected in the terminal ileal tissue at 24-week gestation compared to full-term infants. Low levels of defensins in preterm infants are associated with increased incidence of intestinal pathology especially necrotizing enterocolitis (NEC) [17].
Intracellular levels of APPs are lower in neonates than in later life: human cathelicidin (LL-37) and bactericidal/permeabilityincreasing protein (BPI) levels are reduced in neonatal whole blood and neutrophils when compared with adults, and BPI deficiency of neutrophils in neonates is associated with reduced bacterial-killing capacity [18].
APP levels are lower in preterm than in full-term infants, including the bloodstream, epithelial surfaces and body fluids [19,20].
This relative deficiency in APPs may contribute to the preterm infant’s increased risk for invasive bacterial infection [17].
Higher levels of APPs including HBD-2 are seen in the blood and body fluids of those with acute infections, such as BSI and respiratory infection [21].
In our study there was positive correlation between the weights, gestational ages and (cord blood HβD2 and follow up HβD2) in preterm with negative correlation between the incidence of LOS and both cord blood and follow up serum HβD2.
There was positive correlation between gestational age, TLC and (cord blood HβD2 and follow up HβD2) and negative correlation between platelets, CRP and the presence of signs of LOS (cord blood HβD2 and follow up HβD2) in late preterm.
There was positive correlation between the weight, gestational age and both cord blood HβD2 and follow up HβD2 and negative correlation between CRP and signs of late onset sepsis (cord blood HβD2 and follow up HβD2) in full term.
This is in agreement with Peter et al., [22] who found that HβD2 levels were lower in preterm compared with term neonates (median, 918 vs 1,883 pg/mL; p = 0.003), as well as in neonates with a BW less than 1,500 g compared with those with a BW more than 1,500 g (median, 880 vs 1,749 pg/mL; p = 0.038). HβD2 serum levels were positively correlated with BW and GA (r = 0.359, p = 0.002 and r = 0.364, p = 0.002, respectively). Variables heterogeneously distributed in the subgroups of preterm and term neonates as gender and mode of delivery did not influence HβD2 level. Body weight was also lower in the LOS subgroup when compared with the non-LOS group (p = 0.047). HβD2 serum levels were significantly lower in preterm infants suffering from LOS compared with those who did not suffer from sepsis (513 vs 1,411 pg/mL; p = 0.006).
Our study also was in agreement with Olbrichet al.23who found that HβD2 serum levels were significantly higher in-term neonates compared with preterm neonates.
APP levels are lower in preterm than in full-term infants. This relative deficiency in APPs may contribute to the preterm infant’s increased risk for invasive bacterial infection [17].
ROC curve analysis of cord HβD2 in prediction of sepsis among early pre term stated that the cutoff point of cord blood level of HβD2 is ≤12 ng/L, and that of late preterm was ≤16.5 ng/ L. In full term, the cutoff point of cord blood level of HβD2 was ≤26 ng/L.
6. Conclusion
Low level of HBD2 in cord blood is accompanied with increased incidence of LOS in neonates especially in preterm and low birth weight neonates than full term.
Cutoff point of cord blood level of HβD2 should be put in consideration in predicting LOS in early, late preterm and in full term neonates.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
The authors read and approved the final manuscript. RA conceived and designed the study and revised the manuscript for important intellectual content. RA and MK analyzed the data and wrote the manuscript, revision and submission. AA conducted the laboratory tests and interpreted them.
Acknowledgments
We express our gratitude to all staff members of our Neonatal Intensive Care Unit.