Very recently, Riva A, et al. reported the immunology of viral hepatitis, and showed the role of C-X-C chemokine 10 (CXCL10), a potent chemoattractant for antiviral T-cells and NK-cells, and dipeptidyl peptidase 4 (DPP4), in influencing the clinical outcome of acute hepatitis C virus (HCV) infection [1].The truncated form of CXCL10 is generated by DPP4, and truncated CXCL10 was associated with failure to achieve spontaneous clearance of acute HCV infection in their study. Increased plasma activity of DPP4 was correlated with the establishment of chronic HCV infection via the generation of a truncated form of CXCL10. In another study, high baseline plasma concentrations of DPP4 were also associated with poor treatment outcome and altered HCV-specific T-cell functionality in a cohort of patients with established chronic HCV infection treated with Peg-interferon α plus ribavirin [2].What made a difference in DPP4 activity between patients developing chronicity and patients who spontaneously resolved HCV infection?
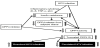
HCV infection induces insulin resistance by increasing inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α (Figure- 1) [3]. DPP4 has a higher release from visceral adipose tissue that is particularly pronounced in obese and insulin-resistant patients [4]. Increased DPP4 activity also induces insulin resistance due to induction of inflammatory cytokines [5, 6].Therefore, I am anxious to know whether a difference in insulin resistance and/or obesity exist or not, between patients developing chronicity and patients who spontaneously resolved HCV infection.
Insulin resistance and diabetes are major disease modifiers in chronic hepatitis C [7].There is further evidence that insulin resistance provides some sort of survival advantage for HCV [7,8].It has been shown that patients who have previously failed anti-viral therapy have greater insulin resistance [8].
These findings suggest that therapeutic inhibition of DPP4 activity by using DPP4 inhibitors may be a novel strategy to treat HCV infection. DPP4 inhibitors reduce DPP4 activity by itself and also via improvement of insulin resistance, resulting in decease in truncated CXCL10, which induces clearance of HCV infection (Figure 1).
Acknowledgments
This work was supported by a grant from the National Center for Global Health and Medicine (25-203).