1. Introduction
Peptic ulcer disease (PUD) is one of the most prevalent gastrointestinal diseases affecting 5-10% of population worldwide [1,2]. Itis characterized by mucosal damage with inflammatory cell infiltration and coagulation necrosis [3]. PUD is a complex disease with multiple contributing factors and unclear etiology [4]. It is believed that PUD is mainly due to imbalance between aggressive and defensive factors affecting gastric mucosa leading to ulcerative damage [3,5]). Pepsin, HCl, reactive free radicals, and refluxed bile are some of aggressive factors [6] while bicarbonate, mucus secretions, mucosal blood flow, nitric oxide (NO), prostaglandins (PGs) as well as enzymatic and non-enzymatic antioxidants are some of the defensive factors [6,7]. Several other factors contribute to the etiology of ulcer progression, for instance, Helicobacter pylori (H. pylori) infection, smoking, excessive use of non-steroidal anti-inflammatory drugs or alcohol, and psychological stress [8,9]. Excessive alcohol consumption apparently raise the risk for major upper gastrointestinal mucosal ulceration and bleeding.
Generally, management of peptic ulcer includes the use of drugs that inhibit gastric acid secretion and/or eradicate H. pylori [9]. However, efficacy of current therapy is not absolute and prolonged use is associated with side effects and ulcer relapse [10,11]. Medicines of plant origin are becoming increasingly favored since a natural substance is expected to promote healing and alleviate illness usually without significant adverse effects [12].
Natural bee honey has been commonly tested as a protective agentinmany experimental model of PUD [13,14]. Similarly, several medicinal plant extracts known for their antioxidant properties were widely investigated as gastroprotective agents [15,16].
Fagoniaindica (family Zygophyllaceae) is a small green spiny under-shrub widely distributed in warm areas of Asia and Africa [17]. Traditionally, this plant has been employed for its anti-inflammatory, antipyretic and analgesic properties [18]. In an earlier study, the authors reported that the plant could be considered as safe and that it contained a variety of bioactive flavonoids, sterols and triterpenoids [19]. The plant is also rich in saponins, tanins and cardiac glycosides [20]. The alcoholic extract of the whole plant was found to exhibit antimicrobial, anti-tumor and analgesic activities. Most of its documented biological activities were found to be attributed mainly to saponin glycosides, triterpenoid saponins, flavonols and rosmarinic acid [19].
The objective of the present investigation was to re-assess, in an animal model, the gastroprotective effects of natural honey solution and to screen for possible protective effects of Fagonia indica alcoholic extract as a gastroprotective non-traditional remedy. Gastroprotective effects were assessed using ulcer index and calculated protective index as well as histopathological examination. Alcohol-induced gastric ulcer in rats was carried out as a model for mimicking the PUD in humans.
2. Material & Methods
2.1 Fagonia indica alcoholic extract
Whole plant was collected during September 2015 from Muhaisna 1 area, Dubai. The plant was kindly identified and authenticated by Prof. Hassnaa Ahmed Hosny, Department of Botany, Faculty of Science, Cairo University, Egypt. Voucher specimens were kept at the Herbarium of the Pharmacognosy Department, Faculty of Pharmacy, Cairo University and in Dubai Pharmacy College, Dubai. The plant was air-dried, powdered (500 g) and then macerated in 70% alcohol. After filtration, the filtrate was evaporated at 50°C using rotary evaporator and the residue (75 g) was collected for the biological study. Extract solution was freshly prepared by dissolving the residue in 1% carboxymethylcellulose (CMC). Acute toxicity study of Fagonia indica alcoholic extract was previously investigated by the authors [19].
2.2 Honey solution
Pure, unprocessed, non-boiled commercial honey obtained from Faculty of Agriculture, Cairo, Egypt was used for this study. Honey was examined, chemically tested and found to be free from inverted sugar, liquid glucose or dextran. For experiments, fresh 10% w/v solution was prepared with distilled water.
3. Animals & Experimental Design
Male Albino rats (n=40), weighing between 220-250g, were obtained from the animal house facility of Dubai Pharmacy College and divided into 4 groups of ten animals each. They were housed under conditions of controlled temperature (23±2°C) and illumination (12 hourslight/ dark cycle) one week prior to and throughout the experimentation period and had free access to water and food. One group was kept as normal without any treatment, while the other three groups were fasted for 28 hours (water was accessible except for the last 2 hours), then administered 80% ethanol solution (0.5 ml of ethanol per each 100g animal body weight) either alone (control ulcer) or with previous treatment with either honey solution (150 mg/kg/day) or Fagonia indica extract (20 mg/kg/day). Animals administered honey solution and the plant extract by oral gavage and continued for 14 days, the last dose being given 1 h before induction of ulcer with ethanol. The normal group received the vehicle (1% CMC) similarly by oral gavage over the two weeks treatment period. All animals’ investigations were performed in accordance with the ethical standards for the proper care and use of laboratory animals and upon approval of the Research Ethical Committee of Dubai Pharmacy College, Dubai, United Arab Emirates.
3.1 Anti-ulcerogenic activity
Rats were sacrificed under ether anesthesia 1 hour after ethanol administration. The stomach was removed and opened along the greater curvature, then rinsed with saline to remove blood clots and gastric contents before being fixed on cardboard to examine the gross gastric lesions. The gastric mucosal layer was carefully inspected using an illuminated magnifying lens for occurrence of ulcers which were measured for their individual lengths. Moreover, each stomach lesions were scored as follows: 0= Normal colored stomach, 0.5= Red coloration, 1= Spot ulcer, 1.5= Streaks < 3 mm length, 2= Streaks ≥ 3 ≤ 5 mm length and finally, 3= Streaks > 5 mm length.
4. Ulcer index, Ulcer Score, and Preventive Index
According to [21], the sum of lengths of all lesions (in millimeters) in each stomach was regarded as the ulcer index (UI). The ulcer score (US) was calculated as the average score of ulcers/stomach in each group [22]. The preventive index of a drug is the percentage inhibition of gastric mucosal damage compared to control untreated results [23]. It is calculated according to the following formula
4.1 Histopathological study
Stomach samples were fixed using 10% formaldehyde for histopathological examination. These tissues were processed and embedded in paraffin wax. Sections of 5 μm in thickness were cut and stained with hematoxylin and eosin [24].
4.2 Statistical analysis
Data were collected using Microsoft Excel, analyzed using SPSS program (version 24) and presented as means + SEM. Comparison between means of each two groups was performed using student's t-test.
5. Result
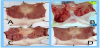
In the present study, the ethanol-induced ulcer model in male albino rats was employed. Oral administration of 80% ethanol to fasting rats caused extensive ulcerations in the gastric mucosa as compared to stomach isolated from normal rats. This is evident by the macroscopic examination of ulceration (Figure 1A & Figure 1B) and the ulcer index in this group was 161.64 ± 14.40 mm as compared to 2.18 ± 0.56 mm in the normal group not receiving ethanol (Figure 2). Moreover, the ulcer score, calculated by the scoring system described in the methodology, revealed a score as high as 46.5 for ethanoltreated rats while the normal rats score was 2 (Figure 2).
Treatment of rats for 14 days with either honey solution or Fagonia indica alcoholic extract was efficient in reducing gastric lesions in the tested doses as demonstrated by the sample pictures of gastric mucosa (Figure 1C & Figure 1D). The treatment with both agents resulted in significantly lower ulcer indices compared to the untreated group (49.47 ± 3.21 mm and 18.22 ± 1.66 mm) for honey-treated and Fagonia indica-treated groups respectively (Figure 2). Gastric protection (calculated preventive index) by honey solution was up to 69% and was significantly greater with Fagonia indica to reach 89%, as shown in the tabulated data of figure 2.
Upon histopathological examination, sections taken from stomach of ethanol-treated animals showed marked hemorrhagic mucosal erosion and sloughing of necrotic cells. The lamina propria is edematous, congested and infiltrated by acute inflammatory cells together with mononuclear cells and hemosiderin-laden macrophages. Areas of hemorrhage are evident (Figure 3A & Figure 3B). On the other hand, sections taken from stomach of honey-treated animals or Fagonia indica treated animals showed minimal focal erosions, mild inflammatory cellular infiltrate, markedly less congestion and edema as compared to ethanol-treated group (Figure 3C & Figure 3D).
6. Discussion
Ethanol is known to damage the gastric mucosa by increasing leukocyte infiltration, decreasing mucosal blood flow and overproduction of free radicals [25]. The dissolution of bicarbonate and mucus by ethanol may also contribute to the deteriorating effect of ethanol [26]. Indeed, in an early study, administration of exogenous prostaglandins have been demonstrated to produce a dose-dependent protection against the gastric mucosal damage induced by alcohol in rats [27].
The assessment of the protective effects of both honey solution and Fagonia indica alcoholic extract, undertaken in the present study, was based on two main criteria; viz., macroscopic examination with the determination of ulcer index, ulcer score and protection index and microscopic examination of stomach specimens from different groups with identification of the histopathological findings.
The protective results obtained for honey solution in the present study against ethanol-induced lesions are in accordance with many previous researches which have demonstrated the gastroprotective activity of several types of honey, either unifloral or multifloral, and from different botanical origin [28,29]. It was demonstrated that honey has significant gastroprotective and antimicrobial properties including inhibition of the growth of H. pylori [13]. An investigation of antiulcer activity of honey in rats concluded that honey restored endogenous antioxidants, retarded lipid peroxidation and preserved mucus production levels through increased production of mucosal glycoproteins and preservation of the gastric mucosal glutathione [14].
The present investigation provides evidence that the alcoholic extract of Fagonia indica possesses a significantly marked degree of gastroprotection against ethanol-induced ulcer in rats. The protection potential of the plant extract was even greater than that of natural honey solution (as shown in the results section). Actually, the plant is widely used in folk and Ayurveda medicine as popular remedy for digestive disorders [30,31]. Since ethanol increases oxidative stress in gastric cells by the formation of free radicals and oxidation of polyunsaturated fatty acids, it is possible that the gastric protection effect of Fagonia indica is a result of its antioxidant effect. In fact, it was previously identified that the ethanolic extract of Fagonia indica contained a high concentration of polyphenolic compounds especially flavonoids from which quercetin was found to be a major [32]. Generally, polyphenolic compounds display a broad spectrum of physiological activities, hence, play a vital role in the prevention of various illnesses including certain kinds of cancer, diabetes, brain dysfunction or other conditions associated with the aging process as well as gastric ulcers [33]. The beneficial role of phenols in protection against mucosal damage was owed to that they stimulate PGE2 formation [34]. Other benefits are attributed to their antioxidant properties through their ability to scavenge free radicals, directly reducing peroxides, and stimulating the antioxidative defense enzyme activities [35]. Flavonoids are among the cytoprotective materials for which anti-ulcerogenic efficacy has been extensively confirmed [36,37]. Researchers demonstrated the protection of alcohol-induced gastric lesions by flavonoids especially quercetin, an element identified in Fagonia indica alcoholic extract [38,39]. Moreover, quercetin and its glycoside increased mucus production, an effect accompanied by a parallel reduction of gastric lesions [40].
7. Conclusion
In conclusion, the protective effect of honey solution is reconfirmed in ethanol-induced gastric ulcer in rats. On the other hand, the significant protective effect of Fagonia indica alcoholic extract is absolutely a new finding of this research work as it was not demonstrated before for this particular species to possess gastroprotective effect in such experimental model. Both honey and Fagonia indica exhibit their beneficial effects probably through antioxidant and mucus production mechanisms, owing to that they are both rich in flavonoids, thus falling in the category of cytoprotective agents. Compared to each other, the beneficial effect of Fagonia indica extract was proven to be significantly higher than that of honey solution as revealed by the gross examination parameters. On the other hand, on a microscopic level, the benefits of both honey solution and the plant extract were equivalent.
Further mechanistic studies are required to unveil molecular mechanism of gastric protection and isolate active constituents of Fagonia indica which might be specifically responsible for the protective action against mucosal damage.
Competing Interests
The authors declare that they have no competing interests.