1. Introduction
The main objective of the study was to evaluate Helicobacter pylori (H pylori) eradication therapy considering eradication regimen, invasive and non-invasive tests performed, and commonly encountered adverse events during eradication therapy in Sultan Qaboos University Hospital (SQUH), a tertiary care teaching hospital.
Helicobacter pylori - formerly called Campylobacter pylori - is a gram-negative rod, spiral-shaped organism with multiple, polar, flagella, it is adapted to life in the stomach. It was first cultured and suggested as a pathogenic factor in peptic ulcer disease by Robin Warren and his colleague Barry Marshall in the early 1980's [1] when they reported the causative evidence linking H pylori to ulceration, which was compelling enough to regard duodenal and gastric ulcers as infectious diseases in the majority of cases. Eradication of H pylori is strongly linked to ulcer healing and prevention of ulcer recurrence [23].
This bacterium survive a highly acidic environment by producing urease, which breaks down urea into ammonia and carbon dioxide and allows the organism to control the pH in its local environment in the stomach by neutralizing the hydrogen ions in gastric acid. It grows in vitro between pH 6 and 8.5 and survives between pH 4 and 8.5 in the absence of urea [4,5]. This may explain the role of proton pump inhibitor being given concomitantly with antibiotics.
Functional differences between strains of H pylori exist based on their genomic pattern. Virulence and tissue damage vacuolating cytotoxin gene (VacA), that causes gastric tissue damage, and cytotoxin-associated gene (CagA) which is not cytotoxic but is antigenic and can be detected serologically [6,7].
Epidemiologic studies have established that more than 50% of the world’s population is colonized with H pylori [4]. According to the International Agency for Research on Cancer, gastric cancer affects 900,000 people annually, the agency classified H pylori as a class 1 carcinogen [8,9].
The American National Institute of Health (NIH) Consensus Panel recommends all patients with duodenal or gastric ulcers and who are H pylori positive should be treated [10]. The European Helicobacter pylori Study Group recognized that many patients presenting to the doctor with symptoms of dyspepsia could be diagnosed and treated in primary care [11].
Acid inhibition combined with antibiotic treatment is effective in eradicating H pylori bacteria. Veldhuyzen et al., [12] compared antimicrobial treatments with conventional ulcer therapy (proton pump inhibitors (PPI) and histamine 2 receptor antagonists (H2RA)) and found that use of antibiotics increases ulcer healing rates from 78 per cent to 93 per cent, decreases first year ulcer recurrence rates from 66 per cent to 9 per cent and eliminates the need for long-term maintenance once the ulcer has healed.
Various regimens of combined anti-secretary agents plus antibiotics have been proposed and tested, but no therapy is 100 per cent effective and no regimen is considered ideal. Many antibiotics show good in vitro activity against H pylori but single antibiotics are usually ineffective in clinical practice and may magnify the problem of drug resistance. This is because of the acidic environment which decreases antibiotic activity, and the protective nature of the gastric mucus in which the bacterium survives [13].
The current standard of H pylori eradication treatment, confirmed by studies presented at the Lisbon workshop of the European meeting on H pylori [14] is seven days of one of three PPI-based triple therapies . These combine a PPI with two antibiotics chosen from clarithromycin, Amoxicillin and metronidazole.
Recently, the sequential triple therapies showed improve in eradication rates, especially with clarithromycin resistant strains. No differences in the prevalence of antimicrobial resistance or incidence of adverse events were observed when comparing sequential to traditional therapy [15,16]. Also, Probiotics, which are live nonpathogenic bacteria, were found to reduce side effects of standard H. pylori treatments as adjunct therapy [17].
Tested H pylori eradication therapies included 7-day, 10-day, and 14-day treatment courses. The duration of therapy is controversial, as shorter periods may enhance compliance but with low eradication rate, on the other hand longer treatment periods favour higher eradication rates and are less likely to be associated with antimicrobial resistance but compliance remains an issue [18,13].
The prevalence of pre-treatment antibiotic resistance varies, but as been estimated at 2-3% for clarithromycin and around 30% for metronidazole [19]. There is particular concern over the emergence of strains resistant to metronidazole [20]. Previous use of metronidazole, particularly for gynaecological infections, may be an important contributory factor [21,22].
H pylori infection is considered eradicated if the organism cannot be detected one month after therapy is stopped. Because urea breath testing (UBT) depends on measuring the presence of urease activity, it is important that suppression of urease activity by medication does not occur at the time of testing, resulting in a false negative result. H2RAs and PPIs are known to produce false-negative urease-based test results [23].
2. Methods
2.1 Study population and sample selection
Study sample was drawn from SQUH a 500-bed governmentfunded tertiary care teaching hospital. All patients aged 18 and above and prescribed H pylori eradication therapy were eligible for this study.
2.2 Sample size
All patients who were prescribed H pylori eradication therapy during a period of 6 months (n=87) were examined. Four patients were excluded because of incomplete information. The rest (n=83) were 64 from surgery and 19 from gastroenterology units.
2.3 Data collection procedure
Data from all prescriptions identified as for H pylori eradication therapy were collected using a predesigned data collection form based on the literature review and expert panel (a clinical pharmacist, a physician and a statistician). Items in the data collection form include patient demographics, endoscopy and diagnosis, regimen structure including does, frequency, duration and maintenance therapy. Patients were interviewed, through telephone calls - after getting their permission - to retrieve the commonly encountered adverse events during H pylori eradication therapy.
To complete the data collection, patients’ clinical records and relevant laboratory tests were reviewed. Pharmacist intervention records were also reviewed to see if changes were made to the prescribed regimens.
2.4 Data analysis
Data analyzed included invasive and non-invasive tests done before and/or after eradication therapy, eradication therapy regimen used adverse events during therapy and demographic characteristics of patients. Eradication therapy regimen was considered inappropriate if the dose, frequency or duration of any of the drugs in a regimen or if the regimen structure was different from the standard eradication therapies based on the British National Formulary (BNF) [24] as per SQUH guidelines. The main outcome of the study was eradication regimen, depending on medications per regimen, dose and frequency of each medication and duration of therapy. Other parameters like endoscopy, non-invasive testing and commonly encountered adverse events during eradication therapy were analyzed based on clinical merit and prior research.
The outcome was compared to the existing European H pylori Study Group and the American College of Gastroenterology guidelines. Eradication therapy regimens were compared to the standard regimens as per the British National Formulary (BNF).
The study was approved by the Research and Ethics committee of the College of medicine & Health Sciences at the Sultan Qaboos University in Muscat, Oman.
[Research proposal MREC 119, Evaluation of H. pylori Eradication Therapy in Dyspeptic Patients in SQUH]
Statistical analyses were conducted using STATA version 12.0 (Stata Corporation, 2007; College Station, TX, USA).
3. Results
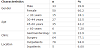
Demographic characteristics of 83 patients (n=83) who were prescribed H pylori eradication therapy during a six-month period are shown in Table 1. Most of patients (60.2%) were females. Patients’ age range was 18 - 70 years and most of them (74.7%) were in the age category of 30 -59 years. The mean age is 43.6 years. Most of the patients (77.1%) were seen and treated by surgeons.
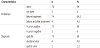
Seventy Eight (94%) of the patients involved in the study were endoscoped. Of those, only four (4.8%) underwent endoscopy before and after treatment. Five patients (6%) were treated empirically with eradication therapy without being tested.
Most of the patient patients (88%) were diagnosed as 'chronic active gastritis' and were H pylori positive. Five patients (6%) of those who underwent endoscopy were H pylori negative. Four patients (4.8%) were endoscopically proven to have duodenal ulcer and concurrent H Pylori infection. Only one patient was reported to have H pylori associated gastric ulcer. He was endoscoped before and after eradication therapy. Table 2 shows clinical characteristics of patients.
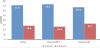
Eradication therapy was considered inappropriate if its structure was different from that of the standard regimens and/or if the dose, frequency or duration of any of the drugs in that regimen was incorrect as per the BNF [24] (Table 3). Thirty-four (41%) of the prescribed regimens were inappropriate. Further analysis of inappropriate regimens revealed incorrect doses, frequencies and duration in 28.6%, 26.5%, and 32.7% of the prescribed medications respectively (Figure 1). Analysis of inappropriate regimens also revealed that 79.4% of the regiments contained one or no antibiotic. Commonly prescribed regimen (24.1%) was the one consists of "Omeprazole 20 mg twice daily + Clarithromycin 500 mg twice daily + Amoxicillin 1 g twice daily” (Table 4). Forty-eight patients (57.8%) were prescribed maintenance therapy, mainly ranitidine (32.5%) or omeprazole (25.3%) as seen in Table 5.
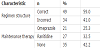
For logistic reasons, only twenty-one patients (25.3%) were interviewed through telephone calls. The commonly encountered adverse event reported by interviewed patients included abdominal pain (37.5%), headache (34.4%), nausea (15.6%), unpleasant or metallic taste (6.3%) and diarrhoea (3.1%). Figure 2 shows graphical presentation of adverse drug event. Only five (23.8%) of the interviewed patients reported that the pharmacist did discuss with them the potential adverse drug reactions and/or pointed out the importance of complying with the prescribed eradication therapy.
4. Discussion
Evidence proved that eradication of H pylori with combined antimicrobial and conventional ulcer therapy (H2RAs or PPIs), while considering patient’s factors, is strongly linked to ulcer healing and prevention of ulcer recurrence [3].
Results of this study revealed that 42.2% of patients were in the age category of 65-59, and this with other symptoms was considered as alarming features. The American Society for Gastroenterology [25] highly recommended endoscopy for patients with alarming features such as the onset of symptoms after 45 years of age, blood loss, weight loss, anaemia, anorexia, and dysphagia.
Most of the patients (94%) in this study were endoscoped before treatment. That was consistent with many guidelines as infection of H pylori can be diagnosed by endoscopic biopsy of the gastric mucosa or by non-invasive methods like Urea Breath Test (UBT) [11,26,27].
Of the eighty-three studied patients, five were offered H pylori eradication therapy despite the negative endoscopy result. This is obviously inappropriate as other causes must be excluded, or if necessary, tests may be repeated to confirm the absence of H pylori. Inappropriate overprescribing of antibiotics would add further to the serious problem of antibiotics resistance among H pylori, and among the normal flora.
According to histopathology reports, 88% of indications for eradication therapy were 'H pylori associated chronic gastritis'. The eradication therapy that considered as inappropriate comprised 41% of the prescribed regimens and almost 80% of these inappropriate regiments contained one or no antibiotic. In general, the selection of appropriate regimen should take into consideration several issues like eradication rate, complexity of regimen, cost, and resistance rate in the population [25].
A standard eradication therapy regimen should comprise a PPI or H2RA and two antibiotics of the following: clarithromycin, amoxicillin and metronidazole [24]. In MACH2 study [28], they found that treatment with omeprazole-clarithromycin-metronidazole or omeprazole-clarithromycin-amoxicillin achieved eradication in 95% and 96% of cases respectively, compared with 79% when omeprazole- amoxicillin-metronidazole regimen was used.
With clarithromycin resistant strains, sequential triple therapy may improve eradication rates. The 10-day regimen involves a PPI (twice daily) and amoxicillin (1 g twice daily) for five days, followed by a PPI (twice daily) plus clarithromycin (500 mg twice daily) and tinidazole (500 mg twice daily) for five days. In one trial, eradication was significantly greater with the sequential regimen than with triple therapy treatment (89 versus 77 percent) [29].
The most prescribed antisecretory drug in this study was omeprazole (43.4%) followed by ranitidine (15.6%), (Table 3). Recent guidelines for H pylori eradication favours PPI over H2RA. Non-availability can influence regimen structure and efficacy of treatment. For example, sometime during this study, prescribers were forced to prescribe ranitidine because omeprazole was not available in our pharmacy.
The synergism observed here is the antisecretory-induced pHdependent increase in the antibiotic sensitivity. Because metronidazole does not depend on cell division in its activity, no such synergism is expected when using PPI plus metronidazole. Furthermore, an antisecretory like PPl markedly inhibits gastric juice volume, thus increasing the concentration of antimicrobials in gastric juice [4,5].
Further analysis of inappropriate regimens showed that 28.6% of doses, 26.5% of frequencies and 32.7% of durations were incorrect. Prescribed doses of Amoxicillin and metronidazole were ranging between 250 mg and 500 mg 8 hourly for a duration of 5-14 days. Clarithromycin was mostly prescribed as 250 mg 6 hourly for 5-14 days. The PPI was commonly prescribed as 20 mg twice daily for up to 30 days. The optimum dose of a macrolide in eradication therapy is still debatable. In a meta-analysis by Huang et al [30]., analysing 26 treatments of one week triple therapies with PPI-clarithromycinmetronidazole, they found that 500 mg of clarithromycin twice a day resulted in a significantly higher eradication rate (90%) than 250 mg twice daily (82%). The combination of PPI-amoxicillin-metronidazole was found less effective than regimens containing a macrolide.
In this study, results revealed 32.7% of prescribed durations as inappropriate in particular with PPI duration which was ranging between 4-8 weeks. As per The European H pylori Study Group, it is not necessary to continue the treatment with PPI plus two antibiotics, for more than two weeks unless there is a complicated ulcer. That could be followed by two to four weeks maintenance therapy with PPI alone [11].
In our hospital. H pylori Resistance to metronidazole reported to be more than 40%. Resistance to antibiotics is considered to be a major cause of failure in the treatment of bacterial infections in general and H pylori infection in particular. When metronidazole-resistant strain of H pylori is present, the effectiveness of eradication therapy can drop to 60%. This is more likely in patients who previously treated with metronidazole [13,20].
Although our local formulary covers all strengths of antibiotics; during this study, amoxicillin was available only as 250 mg capsules (the required daily dose is 1 g 12 hourly) and clarithromycin as 250 mg capsules (the required daily dose is 500 mg 12 hourly). This means that the patient needs to take more than 12 tablets/ capsules per day. Many patients find it difficult to take this amount of tablets/capsules on daily basis. Such issues directly affect compliance and thus the outcome of eradication therapy [13].
This non-availability/shortage problem could be attributed to the location of Oman being far from our main sources of drug supply (UK, USA), but the element of stock control deficiencies cannot be excluded and it could be the reason behind such interruption. Drug shortages are expected to be temporary or involve only a specific strength that has a substitute.
Because of logistic reasons, only a small sample of patients was interviewed to identify the common adverse drug reactions (ADRs) encountered by patients while on eradication therapy. Abdominal pain (37.5%) appeared to be the most common ADR in particular in those who used metronidazole. Other common ADRs included headache (34.4%) and nausea (15.6%). Side effects are reported in up to 50 percent of patients taking one of the triple/quadruple therapy regimens and may stop treatment due to these side effects [31].
Only five (23.8%) of the interviewed patients reported that the pharmacist did discuss with them the potential adverse drug reactions and/or pointed out the importance of complying with the prescribed therapy. According to Graham et al. [32] eradication with triple therapy fell from 96% in patients who took more than 60% of their medication to 69% in those who took less than 60% of their medication. Furthermore, eradication success was 80% in patients who were compliant with medication compared to 54% in those who were not compliant [13].
5. Limitations of Study
Sample size is relatively small and only few patients (25.3%) were interviewed to identify the common ADRs of medications and extent of counselling on eradication therapy.
6. Conclusion and Recommendations
Prescribing pattern for H pylori eradication therapy including regimen structure, doses, frequencies and duration of treatment was evaluated. Significant inappropriate prescribing for treatment and/or maintenance therapy was observed. Furthermore, patients were not fully aware of adverse drug reactions of eradication therapy.
Emphasis should be placed on guidelines as well as counselling to help patients understand the negative consequences of poor adherence to eradication therapy.
Ethical Approval
I would like to add that this study was approved by the Research and Ethics committee of the College of medicine & Health Sciences at the Sultan Qaboos University in Muscat, Oman: [Research proposal MREC 119, Evaluation of H. pylori Eradication Therapy in Dyspeptic Patients in SQUH].
Competing Interests
The author(s) declare that they have no competing interests.